To download or print this page in a different language, choose your language from the drop down menu in the upper left of the website first. If you would like the references to print along with the position paper, please click the “References” area at the bottom of the article to expand them into view and then click the print button.

IAOMT Position Paper on Human Jawbone Cavitations
Chair of the Jawbone Pathology Committee: Ted Reese, DDS, MAGD, NMD, FIAOMT
Karl Anderson, DDS, MS, NMD, FIAOMT
Patricia Berube, DMD, MS, CFMD, FIAOMT
Jerry Bouquot, DDS, MSD
Teresa Franklin, PhD
Jack Kall, DMD, FAGD, MIAOMT
Cody Kriegel, DDS, NMD, FIAOMT
Sushma Lavu, DDS, FIAOMT
Tiffany Shields, DMD, NMD, FIAOMT
Mark Wisniewski, DDS, FIAOMT
The committee would like to express our appreciation to Michael Gossweiler, DDS, MS, NMD, Miguel Stanley, DDS and Stuart Nunally, DDS, MS, FIAOMT, NMD for their critiques of this paper. We also wish to recognize the invaluable contributions and effort made by Dr. Nunnally in compiling the 2014 position paper. His work, diligence and practice provided the backbone for this updated paper.
Approved by the IAOMT Board of Directors September 2023
Table Of Contents
Cone beam computed tomography (CBCT)
Biomarkers and Histological Examination
Evolving Considerations for diagnostic purposes
Acupuncture Meridian Assessment
Systemic and Clinical Implications
Alternative Treatment Strategies
Appendix I IAOMT Survey 2 Results
Appendix II IAOMT Survey 1 Results
Appendix III Images
Figure 1 Fatty degenerative osteonecrosis of the jawbone (FDOJ)
Figure 2 Cytokines in FDOJ compared to Healthy Controls
Figure 3 Surgical procedure for a retromolar FDOJ
Figure 4 Curettage and corresponding x-ray of FDOJ
Movies Video clips of jawbone surgery in patients
References
Over the past decade there has been a growing awareness among the public and health care providers of a link between oral and systemic health 1. For example, periodontal disease is a risk factor for both diabetes and cardiac disease 2. A potentially consequential and increasingly researched link has also been shown between jawbone pathology and the overall health and vitality of the individual 3. The use of technically advanced imaging modalities such as cone-beam computed tomography (CBCT) have been instrumental in identifying jawbone pathologies, which have led to improved diagnostic capabilities 4 and an improved ability to assess the success of surgical interventions 5. Scientific reports, docudramas and social media have increased public awareness of these pathologies, especially among those individuals suffering from unexplained chronic neurological or systemic conditions that fail to respond to traditional medical or dental interventions.
The International Academy of Oral Medicine and Toxicology (IAOMT) is founded upon the belief that science should be the basis upon which all diagnostic and treatment modalities be chosen and utilized. It is with this priority in mind that we 1) provide this update to our 2014 IAOMT Jawbone Osteonecrosis Position Paper, and 2) propose, based on histological observation, a more scientifically and medically accurate name for the disease, specifically, Chronic Ischemic Medullary Disease of the Jawbone (CIMDJ). CIMDJ describes a bone condition characterized by the death of cellular components of cancellous bone, secondary to an interruption of the blood supply 6. Throughout its history, what we are referring to as CIMDJ has been referred to by a multitude of names and acronyms that are listed in Table 1 and will be briefly discussed below.
The goal and intent of this Academy and paper is to provide science, research, and clinical observations for patients and clinicians to make informed decisions when considering these CIMDJ lesions, which are often referred to as jawbone cavitations. This 2023 paper was crafted in a joint effort that included clinicians, researchers and an eminent jawbone pathologist, Dr. Jerry Bouquot, following review of over 270 articles.
In no other bone is the potential for trauma and infections as great as in the jawbones. A review of the literature relating to the topic of jawbone cavitations, (i.e., CIMDJ) shows that this condition has been diagnosed, treated and researched since the 1860’s. In 1867, Dr. H.R. Noel provided a presentation entitled A lecture on caries and necrosis of bone at the Baltimore College of Dental Surgery, and in 1901 jawbone cavitations are discussed at length by William C. Barrett in his textbook entitled, Oral Pathology and Practice: A Textbook for the Use of Students in Dental Colleges and a Handbook for Dental Practitioners 7, 8. G. V. Black, often referred to as the father of modern dentistry, included a section in his 1915 textbook, Special Dental Pathology, to describe ‘the usual appearance and treatment of’ what he described as jawbone osteonecrosis (JON) 9.
Research on jawbone cavitations seemed to come to a halt until the 1970s when others began researching the topic, using a variety of names and labels, and publishing information regarding it in modern oral pathology textbooks 10,11. For example, in 1992 Bouquot et al observed intraosseous inflammation in patients with chronic and severe facial pain (N=135) and coined the term ‘Neuralgia-inducing Cavitational Osteonecrosis’, or NICO 12. Although Bouquot et al did not comment on the etiology of the disease, they concluded that it was probable that the lesions induced a chronic facial neuralgia with unique local features: intraosseous cavity formation and long-standing bone necrosis with minimal healing. In a clinical study of patients with trigeminal (N=38) and facial (N=33) neuralgia, Ratner et al, also showed that almost all of the patients had cavities in alveolar bone and jawbone. The cavities, sometimes more than 1 centimeter in diameter, were at the sites of previous tooth extractions and were generally not detectable by x-rays 10.

 A variety of other terms for what we identify as CIMDJ exist in the literature. These are listed in Table 1 and discussed briefly here. Adams et al coined the term Chronic Fibrosing Osteomyelitis (CFO) in a 2014 position paper 13. The position paper was a result of a multidisciplinary consortium of practitioners from the fields of Oral Medicine, Endodontics, Oral Pathology, Neurology, Rheumatology, Otolaryngology, Periodontology, Psychiatry, Oral and Maxillofacial Radiology, Anesthesia, General Dentistry, Internal Medicine, and Pain Management. The focus of the group was to provide an interdisciplinary platform to treat maladies associated with the head, neck, and face. Through the collective efforts of this group, extensive literature searches and patient interviews, a distinct clinical pattern emerged, which they referred to as CFO. They noted that this disease is often undiagnosed because of its co-morbidities with other systemic conditions. This group pointed out the potential links between the disease and systemic health issues and the need for a team of physicians to properly diagnose and treat the patient 13.
A variety of other terms for what we identify as CIMDJ exist in the literature. These are listed in Table 1 and discussed briefly here. Adams et al coined the term Chronic Fibrosing Osteomyelitis (CFO) in a 2014 position paper 13. The position paper was a result of a multidisciplinary consortium of practitioners from the fields of Oral Medicine, Endodontics, Oral Pathology, Neurology, Rheumatology, Otolaryngology, Periodontology, Psychiatry, Oral and Maxillofacial Radiology, Anesthesia, General Dentistry, Internal Medicine, and Pain Management. The focus of the group was to provide an interdisciplinary platform to treat maladies associated with the head, neck, and face. Through the collective efforts of this group, extensive literature searches and patient interviews, a distinct clinical pattern emerged, which they referred to as CFO. They noted that this disease is often undiagnosed because of its co-morbidities with other systemic conditions. This group pointed out the potential links between the disease and systemic health issues and the need for a team of physicians to properly diagnose and treat the patient 13.
Jawbone cavitational lesions have also been observed in children. In 2013, Obel et al described lesions in children and coined the term Juvenile Mandibular Chronic Osteomyelitis (JMCO). This group suggested the possible use of intravenous (IV) bisphosphonates as treatment for these children. In 2016 Padwa et al published a study describing a focal sterile inflammatory osteitis in pediatric patients’ jawbones 14. They labeled the lesion Pediatric Chronic Nonbacterial Osteomyelitis (CNO).
Since 2010, Dr. Johann Lechner, the most widely published author and researcher on jawbone cavitational lesions, and others have been researching the relationship of these lesions to cytokine production, especially the inflammatory cytokine RANTES (also known as CCL5). Dr. Lechner has used various terms to describe these lesions which have included the previously mentioned NICO but also Aseptic Ischemic Osteonecrosis in the Jawbone (AIOJ), and Fatty Degenerative Osteonecrosis of the Jawbone (FDOJ) 3, 15, 16, 17 . His description/label is based on the physical appearance and/or the macroscopically pathological condition being observed clinically or intraoperatively.
There is now a need to clarify another more recently identified jawbone pathosis which is distinct from the topic of this paper but could be confusing to those researching cavitational lesions. These are bony lesions of the jaw that arise as a result of the use of pharmaceuticals. The lesions are characterized best by loss of blood supply with subsequent uncontrollable sequestration of bone. These lesions have been termed Oral Ulceration with Bone Sequestration (OUBS) by Ruggiero et al in a position paper for American Association of Oral and Maxillofacial Surgeons (AAOMS), as well as by Palla et al, in a systematic review 18,19. Since this problem is related to the use of either one or multiple pharmaceuticals, the IAOMT is of the mindset that this type of lesion is best described as Medication-related Osteonecrosis of the Jaw (MRONJ). MRONJ will not be discussed in this paper as its etiology and treatment approaches are different from that of what we are referring to as CIMDJ, and it has been previously extensively studied 18, 20, 21, 22.
The increasingly common use of Cone-beam computed tomography (CBCT) radiographs by many dental practitioners has led to an increase in the observance of the intramedullary cavitations that we refer to as CIMDJ, and that were previously overlooked and hence ignored. Now that these lesions and anomalies are more readily identified, it becomes the responsibility of the dental profession to diagnose the disease and provide treatment recommendations and care.
Appreciating and identifying the existence of CIMDJ is the starting point of understanding it. Regardless of the many names and acronyms that have been associated with the pathology, the presence of necrotic, or dying bone in the medullary component of the jawbone is well established 3, 12, 23.
When observed during surgery these bony defects present themselves in numerous ways. Some practitioners report that over 75% of lesions are completely hollow or filled with soft, grayish-brown and demineralized/ granulomatis tissue, often with yellow oily material (oil cysts) found in the defective areas with surrounding normal bone anatomy 24. Others report the presence of cavitations having varying overlying cortical bone density that upon opening, appear to have linings with fibrous black, brown or grey filamentous materials 10. Still others report gross changes variously described as “gritty”, “like sawdust”, “hollow cavities”, and “dry” with occasional sclerotic, tooth-like hardness of the cavity walls 12. Upon histological examination, these lesions appear similar to the necrosis that occurs in other bones of the body and are histologically different from osteomyelitis (See Figure 1) 12. Additional images illustrating CIMDJ disease, some that are graphic in nature, are included in Appendix III at the end of this document.
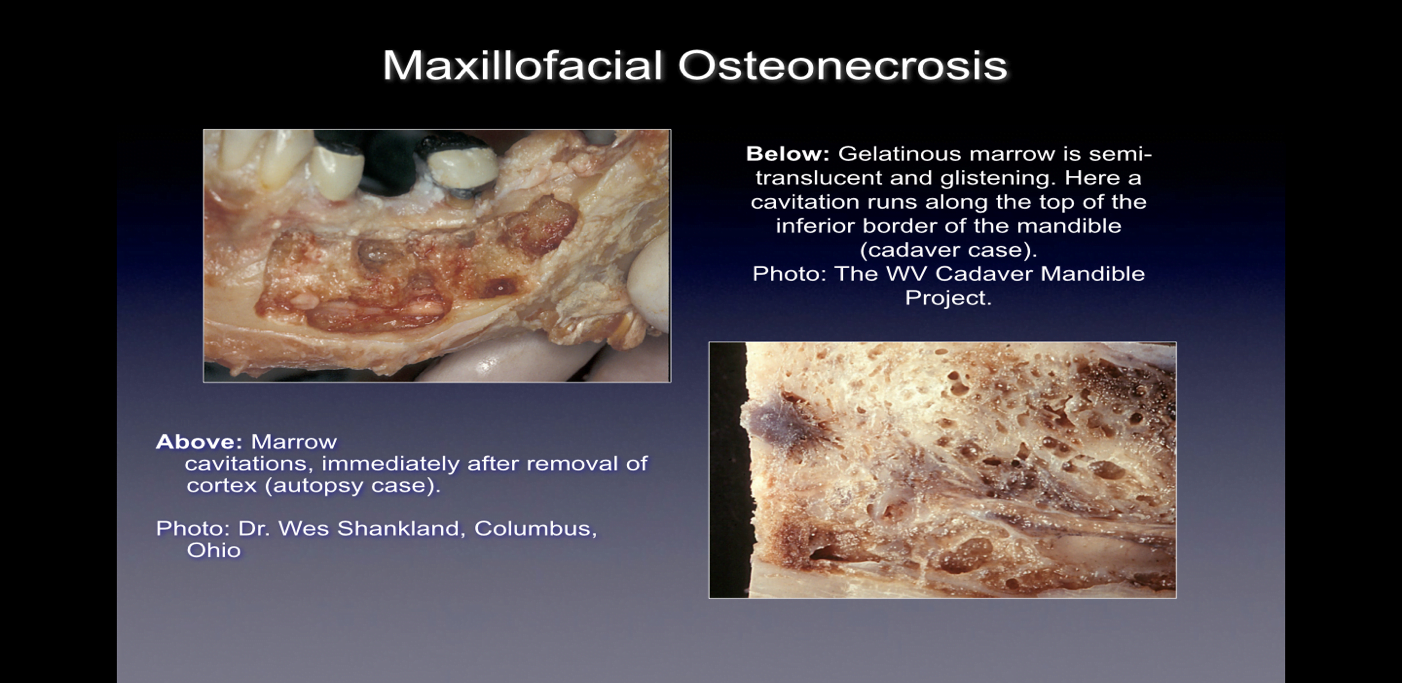
Figure 1 Images of CIMDJ taken from a cadaver
Like other healthcare practitioners, dentists use an organized process that utilizes various methods and modalities to diagnose cavitational lesions. These may consist of conducting a physical examination that includes taking a health history, evaluating symptoms, obtaining body fluids to conduct laboratory tests, and obtaining tissue samples for biopsy and for microbiological testing (i.e., testing for the presence of pathogens). Imaging technologies, such as CBCT are also often used 13. In patients with complex disorders that don’t always follow a pattern or fit a typical order of a symptom complex, the diagnostic process can require a more detailed analysis which at first might only result in a differential diagnosis. Brief descriptions of several of these diagnostic modalities are provided below.
Cone beam computed tomography (CBCT)
Diagnostic techniques described as early as 1979 by Ratner and colleagues, utilizing digital palpation and pressures, diagnostic local anesthetic injections, consideration of medical histories and location of radiating pain are useful in diagnosing jawbone cavitations 10,25. However, while some of these lesions cause pain, swelling, redness and even fever, others do not 26. Thus, a more objective measure, such as imaging is often necessary.
Cavitations are usually not detected on standard two dimensional (2-D such as, periapical and panoramic) radiographic films that are commonly used in dentistry. Ratner and colleagues have shown that 40% or more of the bone needs to be altered to show changes 10, and this is supported by later work 27, and illustrated in Figure 2. This is related to the inherent limitation of 2-D imaging that causes superimposition of anatomical structures, masking areas of interest. In the case of defects or pathology, specifically in the mandible, the masking effect of the dense cortical bone on the underlying structures can be significant 4,28. Therefore, technologically advanced imaging techniques such as CBCT, Tech 99 scans, magnetic resonance imaging (MRI), or trans-alveolar ultrasound sonography (CaviTAU™®) are required 15.
Of the various imaging techniques that are available, the CBCT is the most widely used diagnostic tool used by dentists involved in diagnosing or treating cavitations, and therefore the one we will discuss in depth. The cornerstone of CBCT technology is its ability to view a lesion of interest in 3 dimensions (frontal, sagittal, coronal). CBCT has proven to be a reliable and accurate method of identifying and estimating the size and extent of intra-bony defects in the jaw with less distortion and less magnification than 2-D x-rays 29.
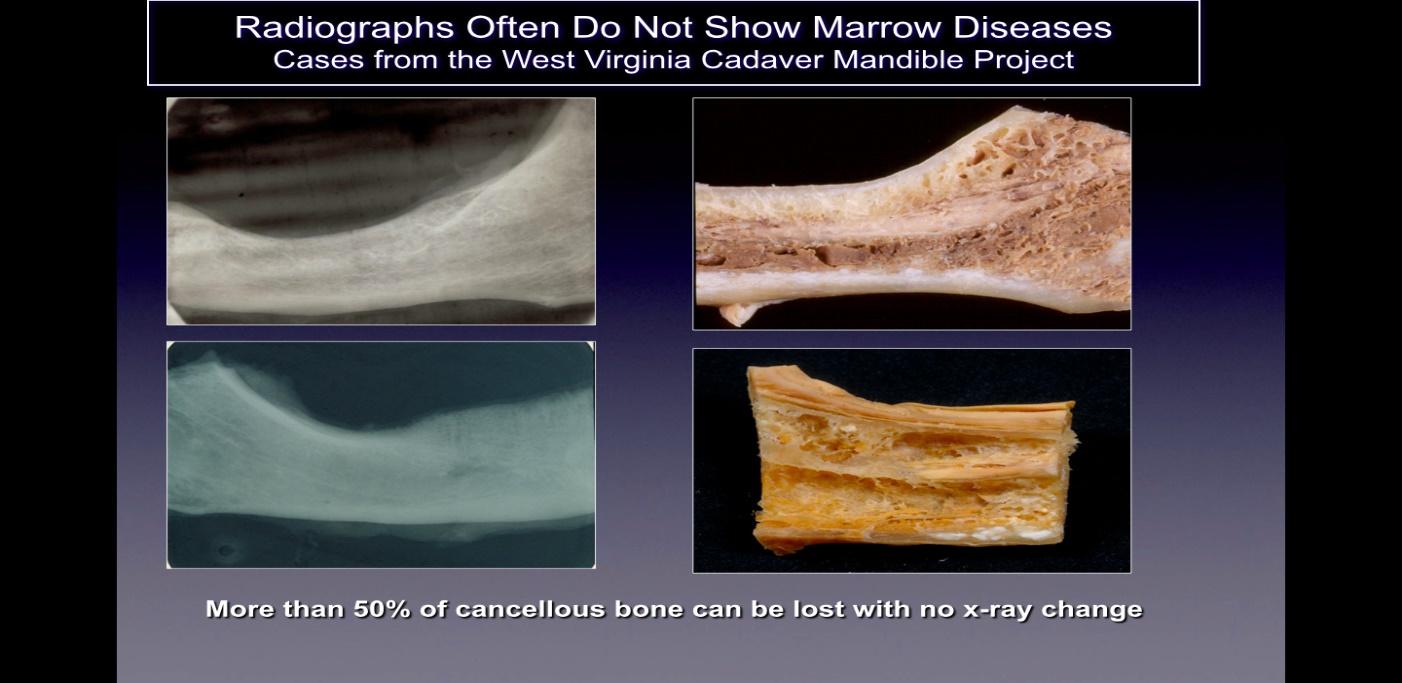
Figure 2 Caption: On the left side are shown 2-D radiographs of jawbones taken from cadavers that appear
healthy. On the right side of the figure are photographs of the same jawbones showing obvious necrotic cavitation.
Figure adapted from Bouquot, 2014.
Clinical studies have shown CBCT images also aid in determining the contents of a lesion (fluid-filled, granulomatous, solid, etc.), possibly helping to distinguish between inflammatory lesions, odontogenic or non-odontogenic tumors, cysts, and other benign or malignant lesions 30,31.
Recently developed software that is specifically integrated with different types of CBCT devices utilizes Hounsfield units (HU) which allows for a standardized assessment of bone density 15,32. HU represent the relative density of body tissues according to a calibrated gray-level scale, based on values for air (-1000 HU), water (0 HU), and bone density (+1000 HU). Figure 3 depicts different views of a modern CBCT image.
To summarize, CBCT has proven useful in the diagnosis and treatment of jawbone cavitations by:
- Identifying the size, extent and 3-D position of a lesion;
- Identifying the proximity of a lesion to other nearby vital anatomical structures such as the
inferior alveolar nerve, maxillary sinus, or adjacent tooth roots;
- Determining the treatment approach: surgery versus non-surgical; and
- Providing a follow-up image to determine the degree of healing and the possible need
to re-treat a lesion.
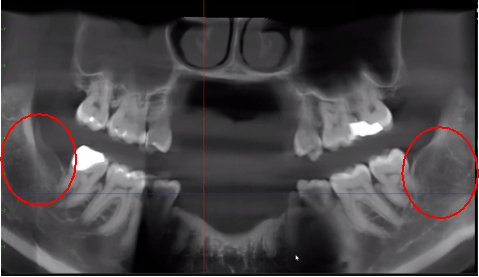
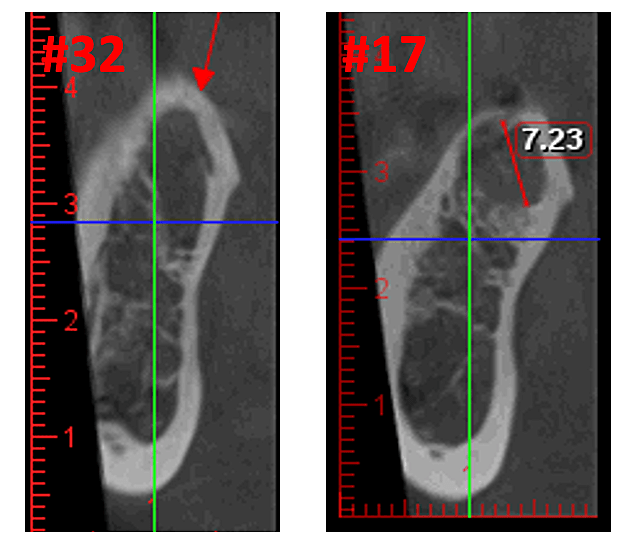
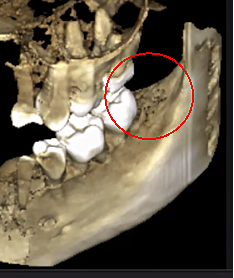
Figure 3 Improved clarity of the CBCT image due to refined software technology, that reduces artifacts and “noise” that dental implants and metal restorations can cause in the image. This allows the dentist and the patient to visualize the lesion more easily. The top panel is a panoramic view of a CBCT showing left (#17) and right (#32) location and extent of the cavitational lesions in a jawbone osteonecrosis patient. The bottom left panel is a saggital view of each site. The bottom right panel is a 3-D rendering of site #17 showing cortical porosity overlying medullary cavitation. Courtesy of Dr. Reese.
We also briefly mention here an ultrasound device, the CaviTAU™®, that has been developed and is being used in parts of Europe, specifically for detecting low bone density areas of the upper and lower jawbones that are suggestive of jawbone cavitations. This trans-alveolar ultrasonic sonography (TAU-n) device is potentially equal in comparison to CBCT in detecting jawbone marrow defects, and has the added benefit of exposing the patient to much lower levels of radiation 15. This device is currently unavailable in the U.S. but is under review by the U.S. Food and Drug Administration and could very well be the primary diagnostic tool used in North America to treat CIMJD.
Biomarkers and Histological Examination
Because of the inflammatory nature of jawbone cavitations Lechner and Baehr, 2017 have investigated the possible relationship between select cytokines and the disease. One cytokine of particular interest is ‘regulated upon activation, normal T-cell expressed and secreted’ (RANTES). This cytokine, as well as fibroblast growth factor (FGF)-2, is expressed in greater amounts in cavitational lesions and in patients with CIMDJ 33. Figure 4, provided by Dr. Lechner, compares the levels of RANTES in patients with cavitations (red bar, left) with the levels in healthy controls (blue bar), showing levels that are more than 25 times greater in those with the disease. Lechner et al uses two approaches to measure cytokine levels. One is to measure the levels of cytokines systemically from blood (Diagnostic Solutions Laboratory, U.S.). A second method is to take a biopsy directly from the diseased site when it is accessed to be evaluated by an oral pathologist. Unfortunately, at this time localized tissue sampling requires complex processing and shipping that is yet to be achieved in non-research facilities, but it has provided insightful correlations.
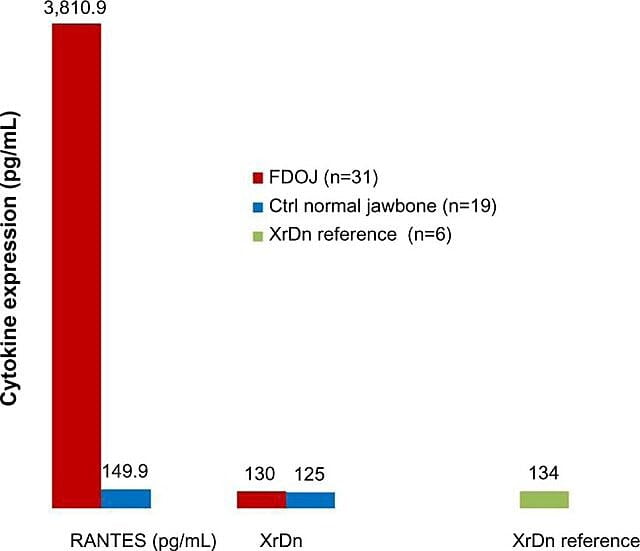
Figure 4 Distribution of RANTES in 31 FDOJ cases and 19 samples of normal jawbone in comparison to an x-ray density reference for both groups in the corresponding areas. Abbreviations: RANTES, regulated upon activation, normal T-cell expressed and secreted chemokine (C-C motif) ligand 5; XrDn, X-ray density; FDOJ, fatty degenerative osteonecrosis of jawbone; n, number; Ctrl, control. Figure provided by Dr. Lechner. License number: CC BY-NC 3.0
Evolving Considerations for diagnostic purposes
The presence of jawbone cavitations has been well established clinically. However, clear diagnoses and best practice treatment parameters need further research. With that in mind it is necessary to briefly mention a few intriguing and potentially valuable techniques that are being used by some practitioners.
It is recognized that additional physiologic assessments would be a valuable screening and diagnostic tool. One such tool being used by some practitioners is thermographic imaging. Generalized inflammatory activity can be seen by measuring heat differentials on the surface of the head and neck. Thermography is safe, rapid and may have diagnostic value similar to that of CBCT 34 . A significant drawback is that it lacks definition, making it difficult to discern the margin or extent of a lesion.
Acupuncture Meridian Assessment
Some practitioners are looking at the energetic profile of a lesion utilizing Acupuncture Meridian Assessment (AMA) to determine its effect on its corresponding energy meridian. This type of assessment is based in Electroacupuncture According to Voll (EAV) 35. This technique, which is based on ancient Chinese medicine and acupuncture principles, has been developed and is being taught in the U.S. 36. Acupuncture has been used to alleviate pain and promote healing 37. It is based on the balance of energy flow (i.e., Chi) through specific pathways of energy in the body. These pathways, or meridians, connect specific organs, tissues, muscles and bones with each other. Acupuncture uses very specific points on a meridian to influence the health and vitality of all the body elements on that meridian. This technique has been used to reveal jawbone disease, that when resolved, also treats seemingly unrelated illnesses, such as arthritis or chronic fatigue syndrome 38. This technique lends itself to further investigation (i.e., results need to be documented and longitudinal data acquired and disseminated).
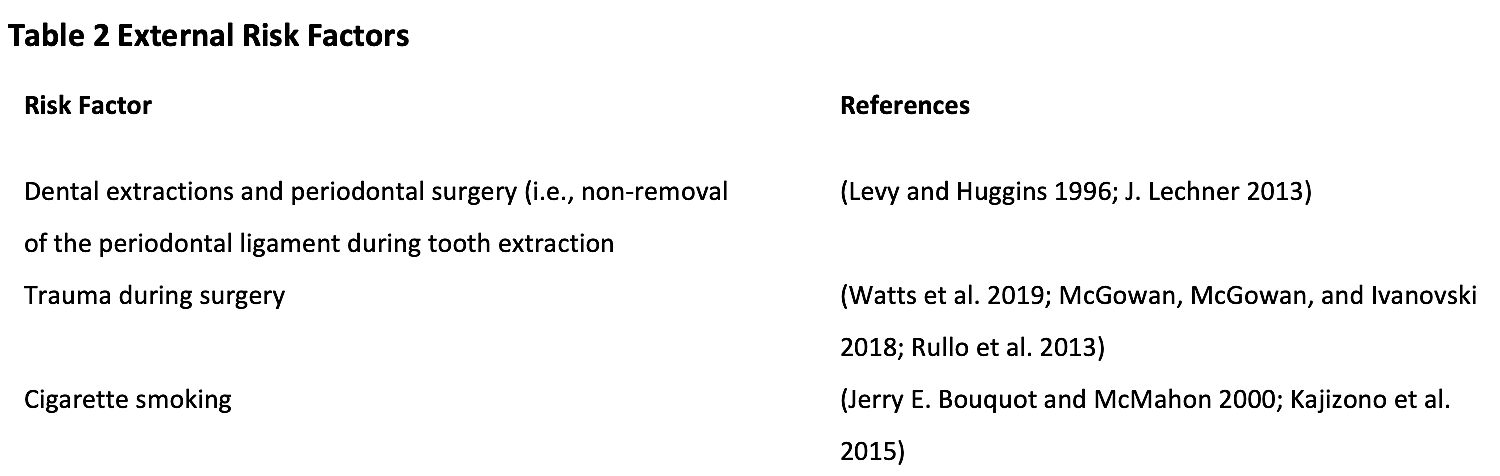
There are many individual factors that increase the risk for the development of jawbone cavitations but usually the risk is multifactorial. Risks to the individual can be either external influences, such as environmental factors or internal influences, such as poor immune function. Tables 2 and 3 list external and internal risk factors.

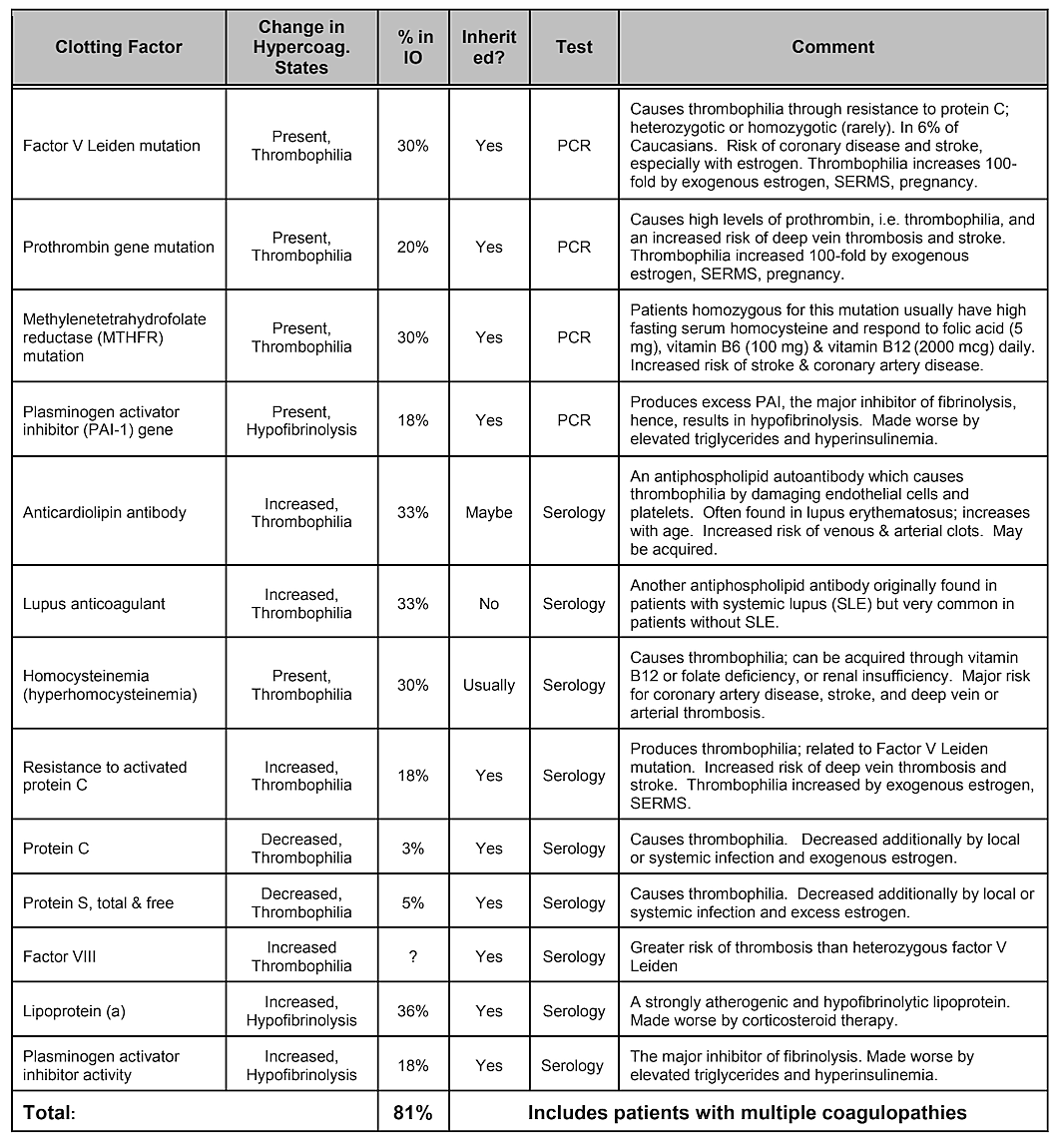
Note that Table 2, Internal Risk Factors, does not include genetic predisposition. While genetic variances would be thought to play a role, no single gene variation or even combination of genes has been shown to be identified as a risk factor, however genetic influences are likely 39. A systematic literature review conducted in 2019 showed that a number of single nucleotide polymorphisms have been identified, but there is no replication across studies. The authors concluded that given the diversity of genes that have shown positive associations with cavitations and the lack of reproducibility of the studies, the role played by genetic causes would appear to be moderate and heterogeneous 40. However, targeting specific populations may be necessary to identify genetic differences 41. Indeed, as has been demonstrated, one of the most common and basic pathophysiologic mechanisms of ischemic bone damage is excess clotting from hypercoagulation states, which usually have genetic underpinnings, as described by Bouquot and Lamarche (1999) 26. Table 4 provided by Dr. Bouquot, lists the disease states that involve hypercoagulation6 and the next 3 paragraphs provide an overview of some of Dr. Bouquot’s findings that he presented in his role as Director of Research at the Maxillofacial Center for Education and Research 6.
In jawbone cavitations there is clear evidence of ischemic osteonecrosis, which is a bone marrow disease wherein the bone becomes necrotic due to oxygen and nutrient deprivation 42. As mentioned, many factors can interact to produce cavitations and up to 80% of patients have a problem, usually inherited, of excessive production of blood clots in their blood vessels 6. This malady is not normally revealed during routine blood tests. Bone is particularly susceptible to this problem of hypercoagulation and develops greatly dilated blood vessels; increased, often painful, internal pressures; stagnation of blood; and even infarctions. This hypercoagulation problem might be suggested by a family history of stroke and heart attacks at an early age (less than 55 years), hip replacement or “arthritis” (especially at an early age), osteonecrosis (especially at an early age), deep vein thrombosis, pulmonary emboli (blood clots in lungs), retinal vein thrombosis (clots in the retina of the eye) and recurrent miscarriage. The jaws have 2 specific problems with this disease: 1) once damaged, the diseased bone is poorly able to withstand low-grade infections from tooth and gum bacteria; and 2) the bone may not recover from the diminished blood flow induced by the local anesthetics used by dentists during dental work. Figure 5 provides a microscopic view of an intravascular thrombus 6.
Table 4 Disease states that involve hypercoagulation. Four out of five jawbone cavitation patients have one of these clotting
factor problems.
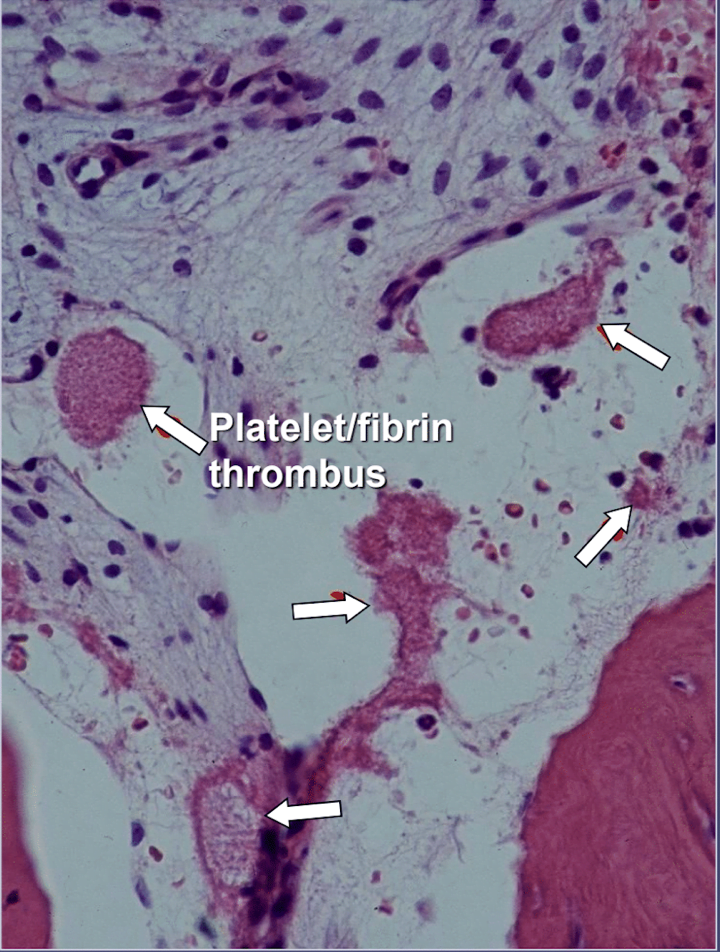
Regardless of the underlying cause of hypercoagulation, the bone develops either a fibrous marrow (fibers can live in nutrient starved areas), a greasy, dead fatty marrow (“wet rot”), a very dry, sometimes leathery marrow (“dry rot”), or a completely hollow marrow space (“cavitation”).
Any bone can be affected, but the hips, knees and jaws are most often involved. Pain is often severe but about 1/3rd of patients do not experience pain. The body has trouble healing itself from this disease and 2/3rds of cases require the surgical removal of damaged marrow, usually by scraping with curettes. Surgery will eliminate the problem (and the pain) in almost 3/4ths of patients with jaw involvement, although repeat surgeries, usually smaller procedures than the first, are required in 40% of patients, sometimes in other parts of the jaws, because the disease so frequently has “skip” lesions (i.e., multiple sites in the same or similar bones), with normal marrow between. More than half of hip patients will eventually get the disease in the opposite hip. More than 1/3rd of jawbone patients will get the disease in other quadrants of the jaw. Recently, it has been found that 40% of patients with osteonecrosis of either the hip or jaw will respond to anticoagulation with low molecular weight heparin (Lovenox) or Coumadin with resolution of pain and with bone healing 6.
Figure 5 Microscopic view of intravascular thrombi
If seeking a non-pharmaceutical approach for reducing the risk for hypercoagulation one can consider the use of supplemental enzymes such as nattokinase or the more powerful lumbrokinase both of which have fibrinolytic and anticoagulation properties 43. In addition, copper deficiency states, which are associated with coagulation dysfunction, should be ruled out because of the increased risk of hypercoagulation observed in patients with jawbone cavitations 44.
Systemic and Clinical Implications
The presence of jawbone cavitations and their associated pathology encompass some specific symptoms but also often include some non-specific systemic symptoms. Thus, its diagnosis and treatment should be approached with thorough consideration by the care team. The most unique and groundbreaking realizations that have come to light since the IAOMT 2014 position paper is the resolution of seemingly unrelated chronic inflammatory conditions following cavitation treatment. Whether systemic illnesses are of an autoimmune nature or inflammation occurring otherwise, significant improvements have been reported, including improvement in cancer. The symptom complex associated with these lesions is highly individualized and therefore not generalizable or easily recognizable. Therefore, the IAOMT is of the mindset that when a patient is diagnosed with jawbone cavitations with or without associated localized pain, and also has other systemic illness previously not attributed to jawbone cavitations, the patient needs further evaluation to determine if the illness is associated with, or is a consequence of the disease. The IAOMT surveyed its members to learn more about what systemic symptoms/illnesses resolve following cavitational surgery. The results are presented in Appendix I.
The presence of cytokines generated in poorly vascularized, necrotic lesions of jawbone cavitations seem to function as a focus of inflammatory cytokines that keep other areas of inflammation active and/or chronic. The relief or at least improvement from localized jaw pain following treatment is hoped for and expected, but this focal theory of inflammation, that will be discussed in detail below, may explain why so many seemingly ‘unrelated’ maladies that have links to chronic inflammatory conditions are also lessened with cavitation treatment 33.
In support of the conclusions drawn in the IAOMT’s 2014 position paper linking jawbone cavitations and systemic illnesses, research and clinical studies more recently published by Lechner, von Baehr and others, show that jawbone cavitation lesions contain a specific cytokine profile not seen in other bone pathologies. When compared with healthy jawbone samples, cavitation pathologies continually show a strong upregulation of fibroblast growth factor (FGF-2), Interleukin 1 receptor antagonist (Il-1ra), and, of particular importance, RANTES 33,45,46. RANTES, also known as CCL5 (c-c motif Ligand 5) has been described as a chemotactic cytokine with a strong proinflammatory action. These chemokines have been shown to interfere in several stages of the immune response and are substantially involved in various pathological conditions and infections 33. Studies have shown RANTES to be implicated in many systemic illnesses such as arthritis, chronic fatigue syndrome, atopic dermatitis, nephritis, colitis, alopecia, thyroid disorders and the promotion of multiple sclerosis and Parkinson’s disease 3,33,45,47. Further, RANTES has been shown to cause an acceleration of tumor growth 3.
Fibroblast growth factors have also been implicated in jawbone cavitations. The Fibroblast growth factors, FGF-2, and their associated receptors, are responsible for many crucial functions, including cell proliferation, survival, and migration. They are also susceptible to being hijacked by cancer cells and playing an oncogenic role in many cancers. For example, FGF-2 promotes tumor and cancer progression in prostate cancer 48. In addition, FGF-2 levels have shown direct correlation to the progression, metastasis and poor survival prognosis in colorectal cancer patients 49. Compared to cancer-free controls, patients with gastric carcinoma have significantly higher levels of FGF-2 in their serum 50. These inflammatory messengers have been implicated in many serious illnesses whether they are of an inflammatory nature or cancerous. In contrast to RANTES/CCL5 and FGF-2, IL1-ra has been shown to act as a strong anti-inflammatory mediator, contributing to the lack of common inflammatory signs within some cavitation lesions 45.
The excessive levels of RANTES and FGF-2 in cavitation lesions have been compared and linked to the levels observed in other systemic illnesses like amyotrophic lateral sclerosis, (ALS) multiple sclerosis (MS), rheumatoid arthritis and breast cancer. Indeed, the levels of these messengers detected in jawbone cavitations are higher than in the serum and cerebrospinal fluid of ALS and MS patients 45. Current research by Lechner and von Baehr has demonstrated a 26-fold increase in RANTES in the jawbone osteonecrotic lesions of breast cancer patients 16. Lechner and colleagues suggest cavitation derived RANTES may serve as an expediter of breast cancer development and progression 16.
As mentioned earlier, there are numerous cases of asymptomatic jawbone cavitations. In these cases, acute pro-inflammatory cytokines such as TNF-alpha and IL-6, are NOT seen in increased numbers in the pathohistological findings of cavitation samples 33,46,51. In these patients, the absence of these pro-inflammatory cytokines is associated with high levels of an anti-inflammatory cytokine Interleukin 1-receptor antagonist (Il-1ra) 52. The reasonable conclusion is that acute inflammation associated with jawbone cavitations is under the control of high levels of RANTES/FGF-2. As a result, to make a diagnosis, Lechner and von Baehr suggest de-emphasizing the focus on the presence of inflammation and consider the signaling pathway, primarily via over expression of RANTES/FGF-2 16. The high levels of RANTES/FGF-2 in cavitation patients indicates that these lesions might be causing similar and mutually reinforcing pathogenic signaling pathways to other organs. The immune system is activated in response to danger signals, which evoke various innate molecular pathways that culminate in inflammatory cytokine production and possible activation of the adaptive immune system. This supports the idea and theory, that jaw bone cavitations may serve as a fundamental cause of chronic inflammatory diseases via RANTES/FGF-2 production 33,53 and further explains why acute symptoms of inflammation aren’t always seen or felt by the patient in the jawbone lesions themselves. Thus, jawbone cavitations and these implicated messengers represent an integrative aspect of inflammatory disease and serve as a potential etiology of the disease. Removing cavitations may be a key to reversing inflammatory diseases. This is supported by the observation of a reduction in serum RANTES levels post-surgical intervention in 5 breast cancer patients (See Table 5) 16. Further research and testing of RANTES/CCL5 levels may provide insight into this relationship. The encouraging observations are the improvements in quality of life realized by many jawbone cavitation patients, whether it be relief at the site of operation or a lessening of chronic inflammation or disease elsewhere.
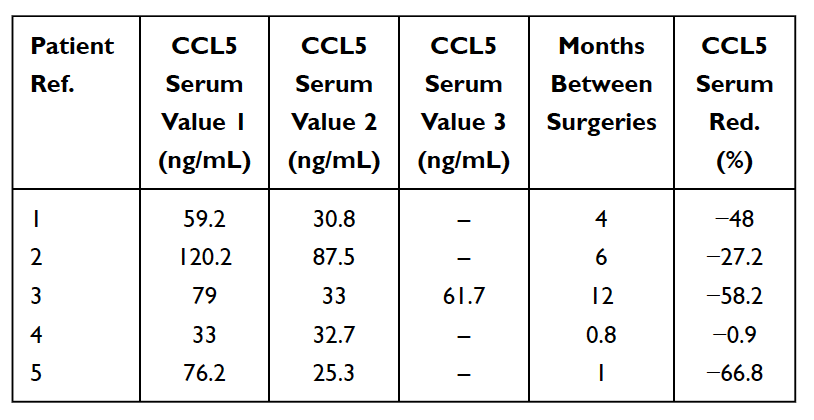
Table 5
Reduction (Red.) in RANTES/CCL5 in serum in 5 breast cancer patients who underwent surgery for fatty-degenerative osteonecrosis of the jawbone (FDOJ). Table adapted from
Lechner et al, 2021. Jawbone Cavitation Expressed RANTES/CCL5: Case Studies Linking Silent Inflammation in the Jawbone with Epistemology of Breast Cancer.” Breast Cancer: Targets and Therapy.
Due to the paucity of literature on the treatment of cavitational lesions, the IAOMT surveyed its membership to gather information regarding what trends and treatments are developing toward a ‘standard of care’. The results of the survey are discussed briefly in Appendix II.
Once the location and size of the lesions are determined, treatment modalities are needed. The IAOMT is of the mindset that it is generally unacceptable to leave “dead bone” in the human body. This is based on data suggesting that jawbone cavitations can be the foci for systemic cytokines and endotoxins to begin the process for degrading a patient’s overall health 2.
Under ideal circumstances a biopsy should be performed to confirm diagnoses of any jawbone pathology and rule out other disease states. Then, treatment to remove or eliminate the involved pathology and stimulate the regrowth of normal, vital bone is necessary. At this time in the peer-reviewed literature, surgical therapy consisting of excising the affected non-vital bone appears to be the favored treatment for jawbone cavitations 54,55. Treatment does involve the use of local anesthetics, which leads to an important consideration. It was previously thought that epinephrine containing anesthetics, which have known vasoconstrictive properties, should be avoided in patients who may already have compromised blood flow associated with their disease state. However, in a series of molecular studies, osteoblastic differentiation increased with the use of epinephrine 56. Therefore, the clinician must determine on a case-by-case basis whether to use epinephrine and if so, the amount that should be used that will give the best results.
Following a surgical decortication and thorough curettage of the lesion 55 and irrigation with sterile normal saline, healing is enhanced by placement of platelet-rich fibrin (PRF) grafts into the osseous void 57–60. The use of platelet-rich fibrin concentrates in surgical procedures is not only beneficial from a clotting standpoint, but also from the aspect of releasing growth factors over a period of up to fourteen days following surgery 61. Prior to the use of PRF grafts and other adjunctive therapies, relapse of the jawbone osteonecrotic lesion after surgery occurred in as many as 40% of cases.
A perusal of the external risk factors outlined in Table 2 strongly suggest that unfavorable outcomes can be avoided with appropriate surgical technique and doctor/patient interaction, especially in susceptible populations. It is advisable to consider adopting atraumatic techniques, minimizing or preventing periodontal and other dental diseases, and choosing an armamentarium that will allow for the best healing outcomes. Providing thorough pre- and post-operative instructions to the patient, including risks associating with smoking cigarettes can help minimize negative outcomes.
Keeping in mind the broad list of potential risk factors listed in Tables 2 and 3, consultations with the patient’s extended care team is recommended to properly ascertain any possible hidden risk factors that may contribute to the development of jawbone cavitations. For example, an important consideration when treating jawbone cavitations is whether the individual is taking antidepressants, specifically selective serotonin reuptake inhibitors (SSRIs). SSRIs have been associated with reduced bone mass density and increased fracture rates 62,63. The SSRI Fluoxetine (Prozac) directly inhibits osteoblast differentiation and mineralization 64. At least two independent studies examining SSRI users compared to controls have shown that SRRI use is associated with worse panoramic morphometric indices 65,66.
Preconditioning may also contribute to successful treatment outcomes. This involves creating a tissue environment conducive to healing by supplying the body with adequate levels of appropriate nutrients that improve the biological terrain by optimizing homeostasis in the body. Preconditioning tactics are not always possible, or acceptable to the patient, but are more important for those patients who have known susceptibilities, such as those with genetic predisposition, healing disorders or compromised health. In such cases, it is critical that this optimization occurs to minimize levels of oxidative stress, which can not only stimulate the disease process but can interfere with the desired healing.
Ideally, the reduction of any toxic load on the body such as fluoride and/or mercury from dental amalgam fillings should be completed before treatment of jawbone cavitations. Mercury can displace iron in the electron transport chain of the mitochondria 67. This results in excess free iron (ferrous iron or Fe++), producing damaging reactive oxygen species (ROS) also known as free radicals, which cause oxidative stress 68. Excess iron in bone tissue also inhibits the proper function of osteoblasts, which obviously will have a negative effect when trying to heal a bone disorder 69.
Other deficiencies should also be addressed prior to treatment. When there is a deficiency of bioavailable copper, magnesium and retinol, metabolism and recycling of iron becomes dysregulated in the body, which contributes to excess free iron in the wrong places leading to even greater oxidative stress and the risk of disease. More specifically, many enzymes in the body (such as ceruloplasmin) become inactive when there are insufficient levels of bioavailable copper, magnesium, and retinol, which then perpetuates systemic iron dysregulation and the resulting increase in oxidative stress and risk of disease 70,71.
Alternative Treatment Strategies
Alternative techniques that are used as primary or supportive therapies should also be evaluated. These include homeopathy, electrical stimulation, light therapy such as photobiomodulation, and laser, medical grade oxygen/ozone, hyperbaric oxygen, anticoagulation modalities, Sanum remedies, nutrition and nutraceuticals, infra-red sauna, intravenous ozone therapy, energy treatments, and others. At this time, the science has not been conducted that would confirm these alternative forms of treatment to be either viable or ineffective. Standards of care to ensure proper healing and detoxification should be established. Techniques for evaluating success should be tested and standardized. Protocols or procedures to help determine when treatment is appropriate and when it is not should be put forth for evaluation.
Research has shown that the presence of jawbone cavitations is an insidious disease process associated with reduced blood flow 25. Compromised medullary blood flow leads to poorly mineralized and inadequate vascularization in areas of the jawbone that can become infected with pathogens, enhancing cellular death. The sluggish blood flow within cavitational lesions challenges the delivery of antibiotics, nutrients and immune messengers. The ischemic environment can also harbor and promote chronic inflammatory mediators that may have even more deleterious effects on systemic health. Genetic predisposition, reduced immune function, effects of certain medications, trauma and infections, and other factors like smoking can instigate or accelerate the development of jawbone cavitations 23.
Along with eminent jawbone pathologist, Dr. Jerry Bouquot, the IAOMT is presenting and promoting a histologically and pathologically correct identification of jawbone cavitational lesions as Chronic Ischemic Medullary Disease of the Jawbone, CIMDJ. Although many names, acronyms, and terms have historically and are currently being used to denote this disease, the IAOMT is convinced that this is the most appropriate term to describe the pathologic and micro-histologic condition commonly found in jawbone cavitations.
Although most jawbone cavitational lesions are difficult to diagnose with routine radiographs and most are not painful, one should never assume that the disease process does not exist. There are many disease processes that are difficult to diagnose, and many that are not painful. If we used pain as an indicator for treatment, periodontal disease, diabetes and most cancers would go untreated. Today’s dental practitioner has a broad spectrum of modalities to successfully treat jawbone cavitations and failure to acknowledge the disease and recommend treatment is no less serious than failure to diagnose and treat periodontal disease. For the health and well-being of our patients, a paradigm shift is crucial for all healthcare professionals, including dental and medical practitioners, to 1) recognize the prevalence of jawbone cavitations and 2) acknowledge the link between jawbone cavitations and systemic illness.
IAOMT SURVEY 2 RESULTS (2023)
As discussed briefly in the paper, unrelated conditions often remit following cavitation surgery. To learn more about what types of conditions resolve and how proximal remission occurs in relation to the surgery, a second survey was sent out to the IAOMT membership. A list of symptoms and conditions that members of this committee have observed to improve after surgery were compiled for the survey. Respondents were asked if they had observed any of these conditions remitting after surgery, and if so to what degree. They were also asked whether the symptoms remitted quickly or if the improvements took longer than two months. Additionally, respondents were queried on whether they typically performed surgery on individual sites, multiple unilateral sites, or all sites in one surgery. The results of the survey are presented in the Figures below. The data are preliminary, given the number of respondents was small (33) and that there is some missing data.
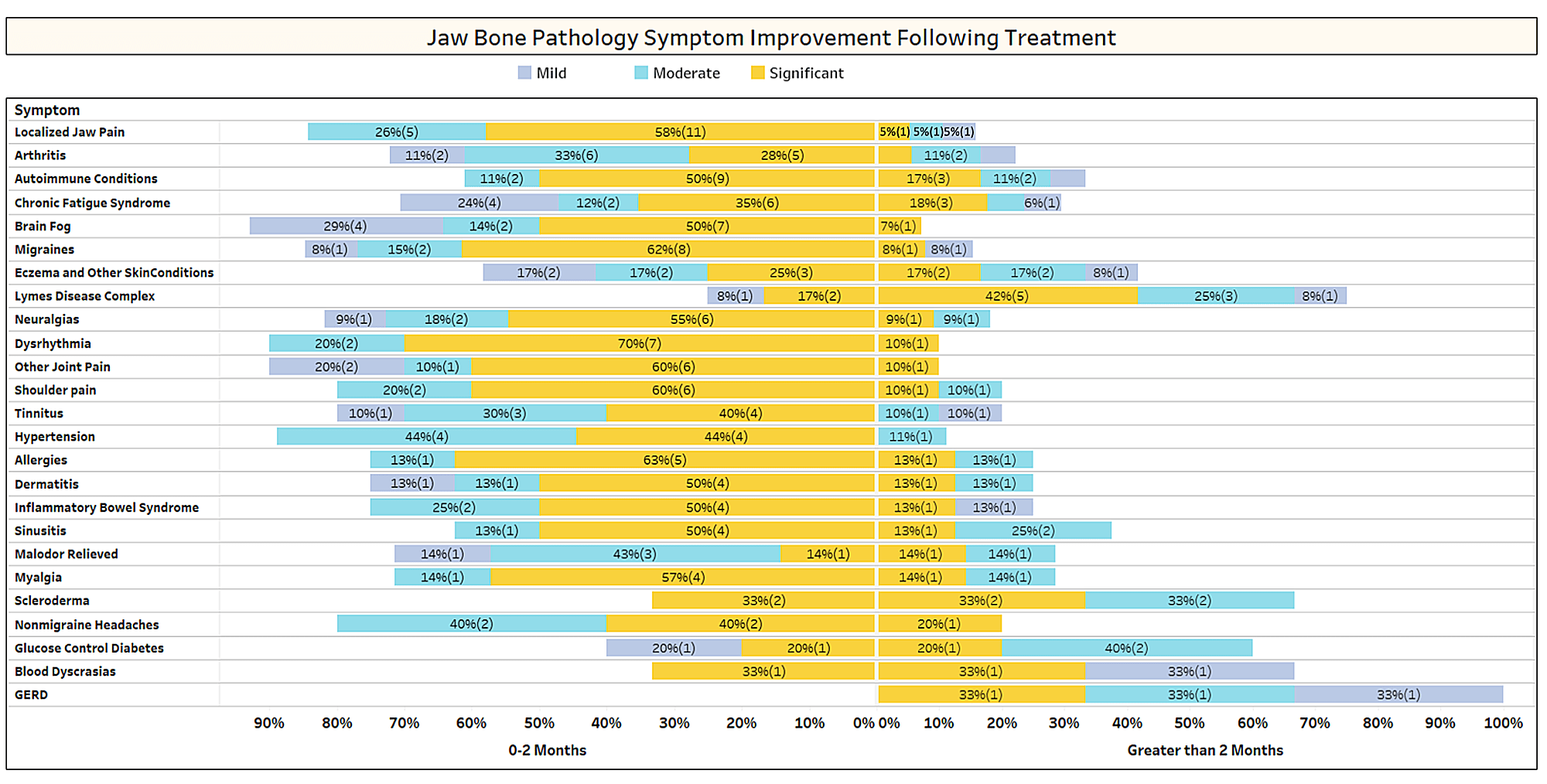
Appx I Fig 1 Respondents rated the level of improvement (mild, moderate or significant) and noted whether improvement occurred rapidly (0-2 months) or took longer (> 2 months). The conditions/symptoms are listed in the order of most reported. Note that most conditions/symptoms remitted in less than two months (left side of the midline).
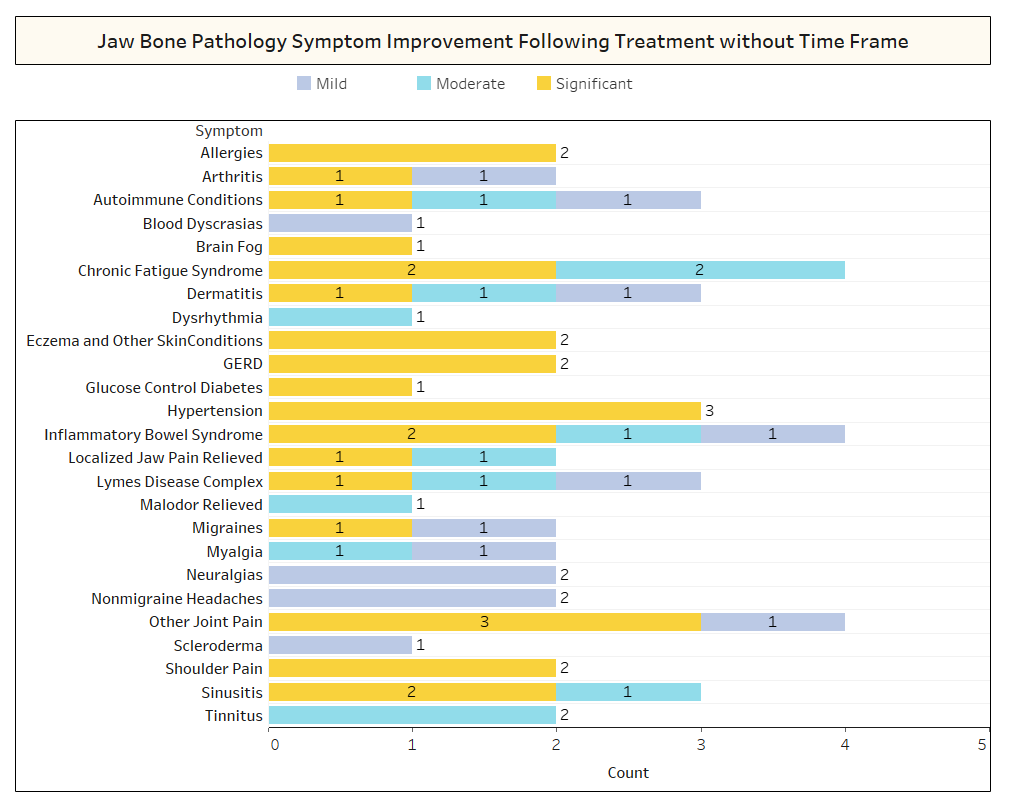
Appx I Fig 2 As shown above, in several cases, Respondents did not note the timeframe of recovery for the improvements that were observed.
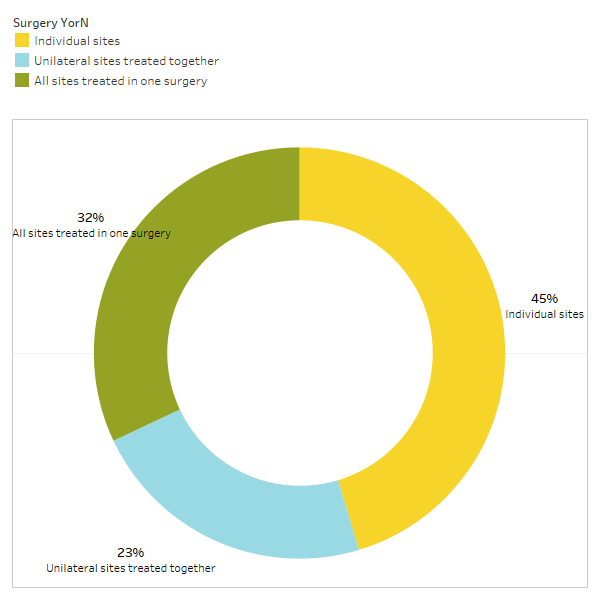
Appx I Fig 3 Respondents responded to the query, “Do you typically recommend/perform
a surgery for individual sites, unilateral sites treated together, or all sites treated in one surgery?”
IAOMT SURVEY 1 RESULTS (2021)
Due to the scarcity of literature and clinical case reviews relating to the treatment of cavitational lesions, the IAOMT surveyed its membership to gather information regarding what trends and treatments are developing toward a ‘standard of care’. The full survey is available on the IAOMT website (note that not all practitioners responded to all survey queries).
To briefly summarize, the majority of the 79 respondents offer surgical treatment, which involves soft tissue reflection, surgical access of the cavitation site, and various methods of physically ‘cleaning out’ and disinfecting the affected site. A wide range of medicaments, nutraceuticals, and/or blood products are used to promote healing of the lesion prior to closing the soft tissue incision.
Rotary burs are often used to open or access the bony lesion. Most clinicians use a hand instrument to curette or scrape out the diseased bone (68%), but other techniques and tools are also utilized, such as a rotary bur (40%), a piezoelectric (ultrasonic) instrument (35%) or a ER:YAG laser (36%), which is a laser frequency used for photoacoustic streaming <sup>72</sup>.
Once the site is cleaned out, debrided, and/or curetted, most respondents use ozone water/gas to disinfect and and promote healing. 86% of the respondents utilize PRF (platelet-rich fibrin), PRP (platelet-rich plasma) or ozonated PRF or PRP. A promising disinfecting technique reported in the literature and within this survey (42%) is the intraoperative use of Er:YAG <sup>72</sup>. 32% of the respondents do not use any type of bone graft to fill in the cavitation site.
Most respondents (59%) typically do not biopsy the lesions stating a variety of reasons from cost, inability to obtain viable tissue samples, difficulty in finding a pathology lab, or certainty of the status of the disease.
Most respondents do not use antibiotics pre-operatively (79%), during surgery (95%) or post-operatively (69%). Other IV support that is used includes dexamethasone steroids (8%) and Vitamins C (48%). Many respondents (52%) utilize low level laser therapy (LLLT) post operatively for healing purposes. Many respondents recommend nutrient support including vitamins, minerals, and various homeopathics prior to (81%) and during (93%) the healing period.
 Images
Images
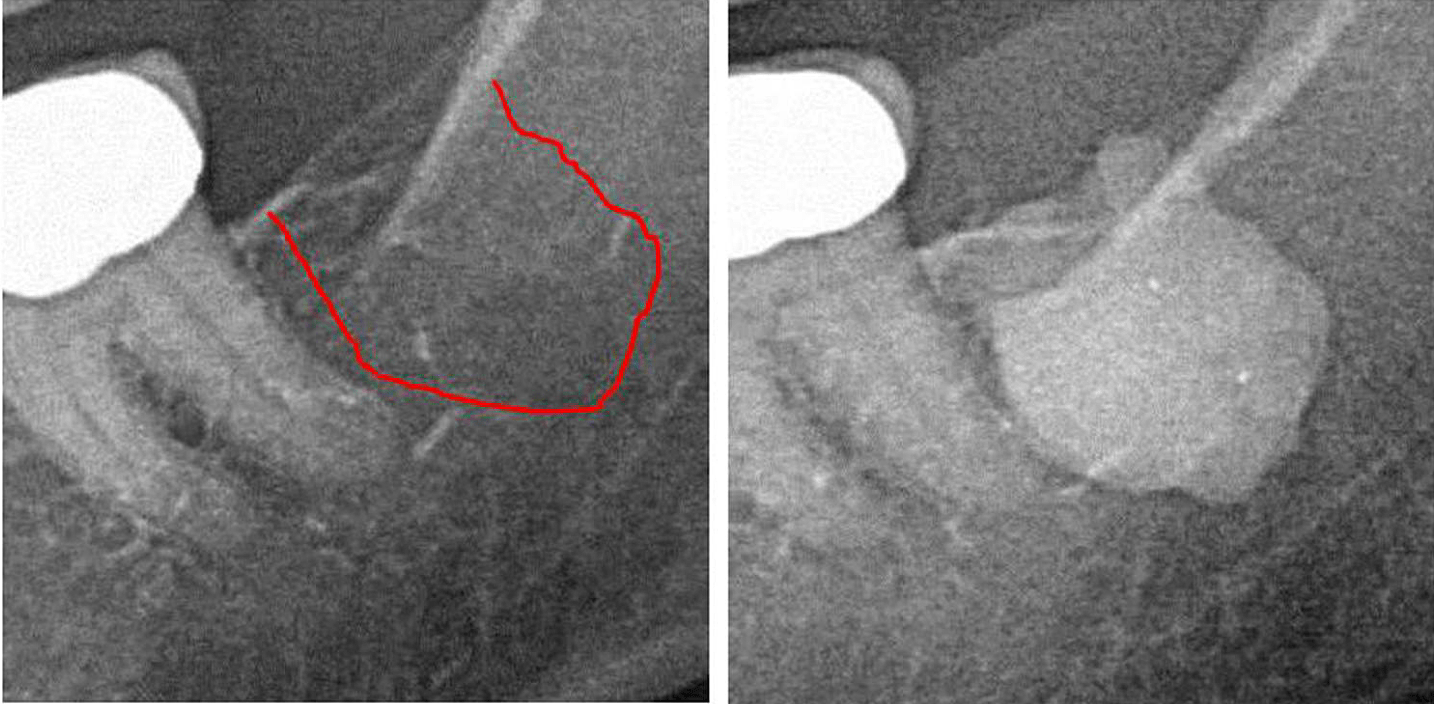
Appx III Fig 1 Left panel: 2D X-ray diagnostics of area #38. Right panel: Documentation of the expanse of FDO) in retromolar area 38/39 using a contrast agent after FDOJ surgery.
Abbreviations: FDOJ, fatty degenerative osteonecrosis of the jawbone.
Adapted from Lechner, et al, 2021. “Jawbone Cavitation Expressed RANTES/CCL5: Case Studies Linking Silent Inflammation in the Jawbone with Epistemology of Breast Cancer.” Breast Cancer: Targets and Therapy
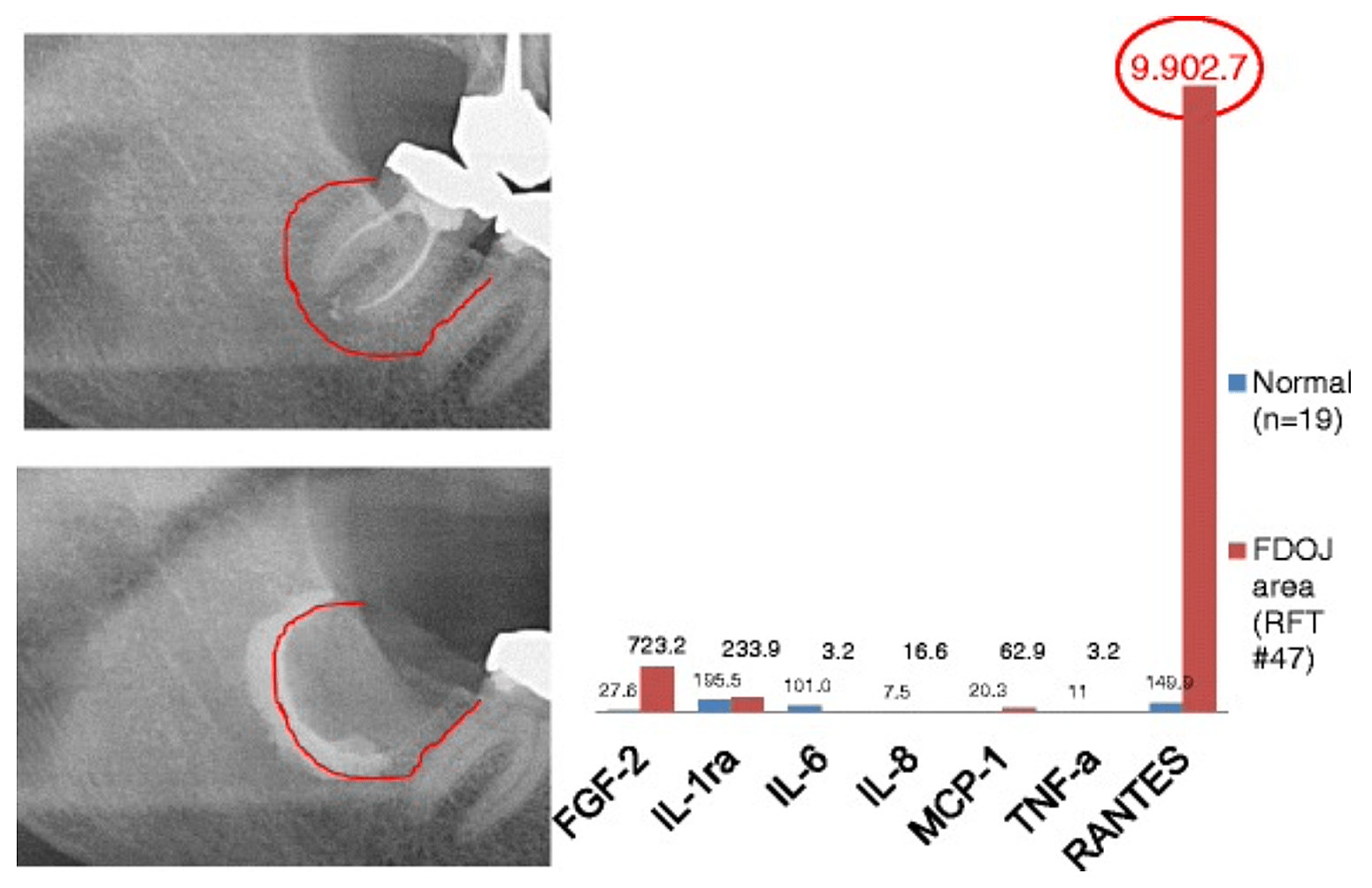
Appx 3 Fig 2 Comparison of seven cytokines (FGF-2, IL-1ra, IL-6, IL-8, MCP-1, TNF-a and RANTES) in FDOJ underneath RFT #47 with the cytokines in the healthy jawbone (n = 19). Intraoperative documentation of extension of FDOJ in the right lower jawbone, area #47 apically of RFT #47, by contrast agent after the surgical removal of RFT #47.
Abbreviations: FDOJ, fatty degenerative osteonecrosis of the jawbone.
Adapted from Lechner and von Baehr, 2015. “Chemokine RANTES/CCL5 as an Unknown Link between Wound Healing in the Jawbone and Systemic Disease: Is Prediction and Tailored Treatments in the Horizon?” The EPMA Journal
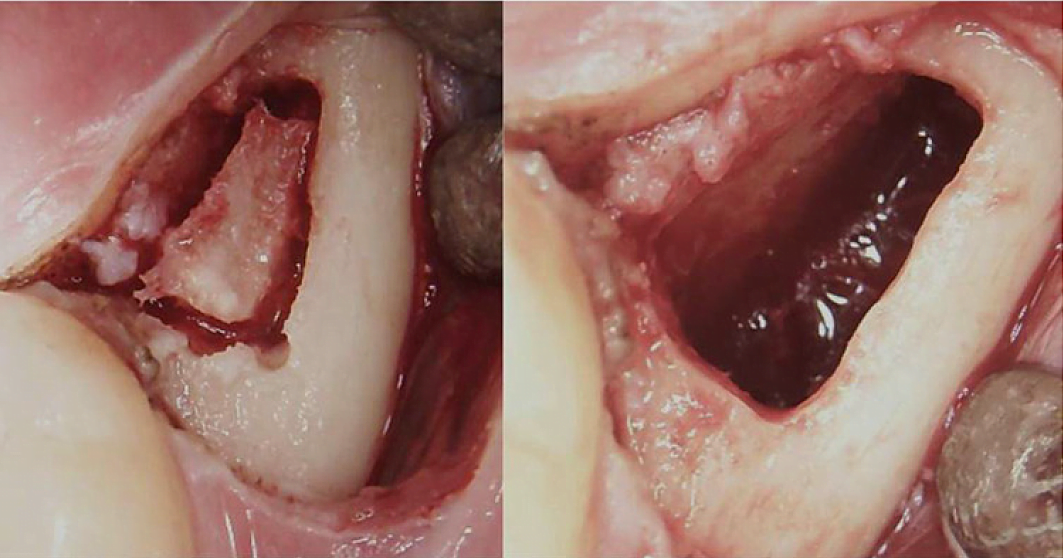
Appx III Fig 3 Surgical procedure for a retromolar BMDJ/FDOJ. Left panel: after folding down the mucoperiosteal flap, a bone window was formed in the cortex. Right panel: curetted medullary cavity.
Abbreviations: BMDJ, bone marrow defect in jawbone; FDOJ, fatty degenerative osteonecrosis of the jawbone.
Adapted from Lechner, et al, 2021. “Chronic Fatigue Syndrome and Bone Marrow Defects of the Jaw – A Case Report on Additional Dental X-Ray Diagnostics with Ultrasound.” International Medical Case Reports Journal
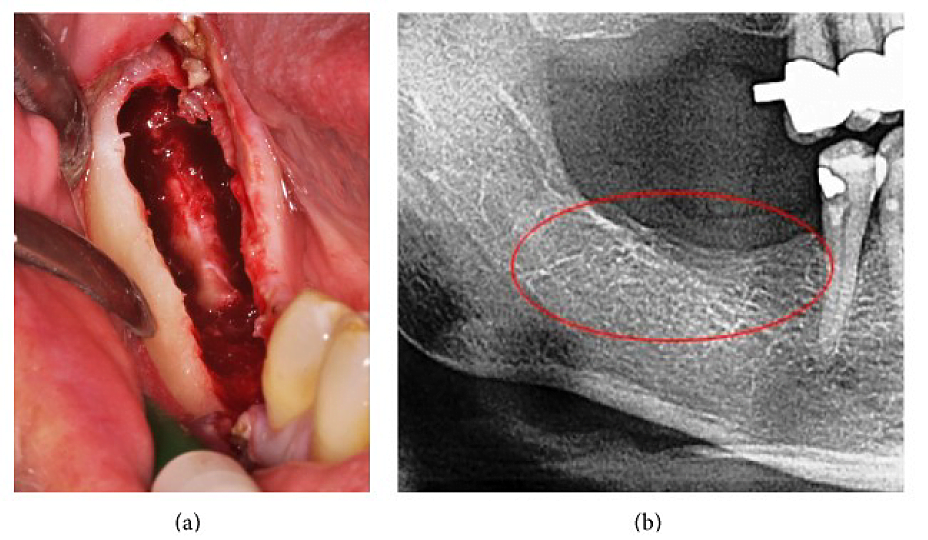
Appx III Fig 4 (a) Curettage of FDOJ in the lower jaw with denuded infra-alveolar nerve. (b) Corresponding X-ray without any signs of pathological process in jawbone.
Abbreviations: FDOJ, fatty degenerative osteonecrosis of the jawbone
Adapted from Lechner, et al, 2015. “Peripheral Neuropathic Facial/Trigeminal Pain and RANTES/CCL5 in Jawbone Cavitation.” Evidence-Based Complementary and Alternative Medicine
Appx III Movie 1
Video clip (double-click on the image to view the clip) of jawbone surgery showing fat globules and purulent discharge from the jawbone of a patient who was suspected of having jawbone necrosis. Courtesy of Dr. Miguel Stanley, DDS
Appx III Movie 2
Video clip (double-click on the image to view the clip) of jawbone surgery showing fat globules and purulent discharge from the jawbone of a patient who was suspected of having jawbone necrosis. Courtesy of Dr. Miguel Stanley, DDS
To download or print this page in a different language, choose your language from the drop down menu in the upper left of the website first. If you would like the references to print along with the position paper, please click the “References” area at the bottom of the article to expand them into view and then click the print button.
IAOMT Position Paper on Human Jawbone Cavitations Authors













