
The Petitioners listed below submit this Petition for Reconsideration pursuant to 21 C.F.R. § 10.33, and hereby request that the Food & Drug Administration formally ban the use of encapsulated mercury fillings as a dental restorative material, or alternatively reclassify dental amalgam fillings from Class II to Class III.
A. Petitioners:
- International Academy of Oral Medicine and Toxicology (“IAOMT”)
- Dental Amalgam Mercury Solutions Inc. (“DAMS INC”)
Citizen’s Petition
The undersigned submits this petition for reconsideration of the decision of the
Commissioner of Food and Drugs in Docket No. ________________.
A. Action Requested:
This Petition pertains to dental mercury capsules (hereinafter referred to as “mercury fillings” or “dental amalgams”). It is hereby requested that the Commissioner of the Food and Drug Administration (FDA) take the following actions with respect to mercury fillings:
1. Formally ban the use of encapsulated mercury fillings as a dental restorative material pursuant to section 516 of the Medical Device Amendments of 1976 (21 U.S.C. § 360f) and 21C.F.R. 895. The risk of illness or injury associated with the use of dental mercury presents an unreasonable, direct and substantial danger to the health of people who have them, as well as the people who place them (i.e., dental personnel).
2. Alternatively, place encapsulated mercury fillings into Class III pursuant to section 513(3) of the Act (21 U.S.C. § 360c(e)) and 21 CFR 860 and seek strict proof of safety and effectiveness.
3. If the FDA decides to place encapsulated mercury fillings into Class III, FDA should place restrictions (not special controls or recommendations) on the use of this material in children ages 0-19, women of childbearing age, people with compromised kidney, immune, and neurological function, those who are hypersensitive to mercury, those who test positive for apolipoprotein E4 or coproporphyrinogen oxidase (CPOX4), and other persons within susceptible subpopulations as described herein. Neither “Class II controls” nor “Special Controls” can accomplish a reasonable assurance of safety for all sectors of our general population. Reasonable assurance of safety can only be achieved by abolishing the use dental amalgam or by placing it into Class III. However, given that only 15% of Americans do not fall into the above categories of risk, banning its use is the only real solution (See Appendix I).
B. BACKGROUND:
Over 122 million Americans, about 1/3 of the population, have mercury amalgam fillings,[1] with millions more placed annually. Those most affected are low-income individuals relying on government aid, including seniors, service members, and veterans. By continuing to allow and support amalgam use, we are forcing these vulnerable groups to receive the cheapest, and most toxic option, without choice.
To reduce mercury exposure, the U.S. must end the use of dental amalgam and reimburse only mercury-free alternatives. Mercury exposure is highest during placement and removal, but even after placement, amalgam continuously emits mercury vapor, especially during eating, chewing, or brushing. Often ignored but important to mention, mercury also off-gases at higher rates when amalgam fillings crack, which often goes unnoticed. This exposure harms human health, as noted by the Minamata Convention. Appendix I highlights recent studies linking chronic mercury exposure from amalgam fillings to serious health issues.
Banning amalgam fillings would not only address associated health risks but also improve dental outcomes and reduce long-term costs. Amalgam requires removal of healthy tooth structure and weakens teeth, often leading to cracks, root canals, or extractions.[2] See Appendix II for multiple lines of evidence clearly showing that composite resin fillings, made of quartz or silicon powder in a resin matrix, are a superior option.
Banning amalgam use will help protect the environment. Around 2,220 metric tons of mercury are emitted annually from human activities,[3] with dental amalgam contributing through air (cremation, clinic emissions), water (wastewater), and soil (landfills, burials). The EPA, recognizing this threat, issued a 94-page rule requiring dental offices using amalgam to install separators,[4] yet only 40% comply. These separators prevent mercury from entering municipal sewage systems, where dental offices are the top mercury source,[5] releasing up to 5.1 tons per year.[6] Although the requirement to install amalgam separators went into effect in July 2020, enforcement is lacking. Dentists only need to submit a one-time compliance report (See Appendix III), with no ongoing monitoring, meaning 60% of dentists who don’t use separators face no consequences. Even when installed, separators alone don’t guarantee mercury control: A study of 12 clinics found that proper maintenance of amalgam separators significantly reduced mercury discharge, from 84 to 6 grams per chair.[7] The EPA states that “Removing mercury when it is in a concentrated and easy to manage form in dental amalgam, before it becomes diluted and difficult and costly to remove, is a commonsense step to prevent mercury from being released into the environment where it can become a hazard to humans.”[8] But is that true? Wouldn’t it be prudent to mandate the use of alternative materials and ban the use of Civil War-era mercury amalgam fillings altogether?
C. HISTORY:
It is important to examine the legal and regulatory failures that have enabled decades of inaction on the matter of dental amalgams and the pressing need for a nationwide ban.
Amalgam restorations have been in use for over 150 years. Due to its long-standing use, dental amalgam was “grandfathered” in, such that it was not subject to premarket testing requirements.
In 1976, Congress mandated the FDA to complete a classification of dental amalgam. In 2009, under pressure from citizen’s lawsuits, the FDA completed the classification and determined that amalgam was harmless to everyone over age 6. It took 33 years for the classification to be completed. However, the classification determination was severely flawed as it ignored the full range of exposures across individuals and did not control for body weight. In other words, a 40-pound child was treated in the analysis in exactly the same manner as a 200-pound 60-year-old man. It also excluded all children under 6 years of age. It also did not control for size of the amalgam filling, a crucial variable. These issues were contested by concerned citizens forcing the FDA to convene an expert panel to reconsider the risk assessment. This is discussed further below.
On August 4, 2009, the FDA ruled for the first time that dental amalgam should be placed in FDA’s Class II. On behalf of the IAOMT and other petitioners, and in response to this ruling, I, James Love, Attorney at Law, prepared a citizen’s petition to the FDA (Citizen Petition Docket No. FDA-2009-P-0357, July 25, 2009) seeking administrative relief, which included the following: discontinuation of the use of mercury amalgam fillings in the following classes of persons: young children, women and particularly women of childbearing age, patients with compromised kidney, immune, and neurological function, those who are hypersensitive to mercury, those who test positive for apolipoprotein E4 or coproporphyrinogen oxidase (CPOX4), and other persons within susceptible subpopulations described in the petition. I argued that “[n]either Class II controls or Special Controls [could] accomplish a reasonable assurance of safety for all sectors of our general population. Reasonable assurance of safety [could] only be achieved by abolishing the use dental amalgam or by placing it into Class III.” [FDA provided an interim response to this petition on January 21, 2010, with no substantive value.]
In response to these and other petitions, the FDA held hearings before a Scientific Advisory Panel in December 2010. The FDA commissioned a team of experts, to examine mercury exposure and the risks associated with the use of dental amalgam. Using the most conservative metric, it was concluded that over 67 million Americans exceed the maximum dose, considered to be safe, established by the US Environmental Protection Agency (EPA).[9] The findings were used as a centerpiece at the FDA’s Expert Panel review. The head scientist, Dr. Richardson, stated, “The proportion of the American population predicted to exceed the U.S. EPA [maximum safe] dose for mercury vapor due to dental amalgam is large and would not be supported or permitted by regulation for other sources of exposure.” The FDA themselves commissioned Dr. Richardson to inform regulation, and yet chose not to act.
As the author of three petitions and the attorney for the IAOMT, I was given a block of time to address this Scientific Advisory Panel, which was mostly ceded to scientists knowledgeable on the topic. At the conclusion of these hearings, Jeffrey Shuren, M.D., J.D., head of the FDA’s Center for Devices and Radiological Health, assured those in attendance that an FDA ruling on these petitions would be forthcoming before the end of 2011.
An FDA response was not provided at end of 2011. By 2014, those who were involved with the FDA petitions and ensuing hearings had given up hope of receiving any response. We learned that the Scientific Advisory Panel privately advised the FDA who reported, futuristically dated “January XX, 2012,” to “consider warnings against the use of dental amalgam in pregnant women, young children, and those with kidney dysfunction, neurological impairment, or allergy to mercury and other components of dental amalgam fillings.” The FDA also states in that report: “However, alternative materials, such as composite resins, that do not contain mercury, can also be used to fill cavities. The FDA believes that these alternative materials would best be offered as the first line of restorative care minimizing the use of amalgam.” (See Appendix II)
FDA officials quietly advised that its parent agency, the Department of Health and Human Services (“HHS”) had discreetly killed FDA’s control of this issue.
The nationally recognized McClatchy DC News described the above activities in depth and included the repressed report on July 21, 2015 (Appendices IV and V). The reporter, Greg Gordon, was aware of the Scientific Advisory Panel’s safety communications to the FDA and aware of HHS’s decision to conceal this communication. Mr. Gordon states: “The proposal and its secret rejection, after a cost-benefit analysis by officials at the Department of Health and Human Services, have put the Obama administration in the awkward position of concealing for over three years a safety communication potentially affecting millions of Americans.”
On behalf of the IAOMT and others, I obtained a Court order compelling an FDA response to the petition. I filed a lawsuit in March 2014 in the U.S. District Court for the District of Columbia seeking to compel such a response. Shortly thereafter, the FDA agreed to prepare a response. The response, dated January 27, 2015, submitted and signed by Leslie Kux, Associate Commissioner for Policy, denied the petition. FDA declined to restrict the use of dental amalgam in any meaningful way, failed to place mercury fillings in Class III, and failed to make meaningful and relevant information available to the public so that dental patients could make truly informed decisions. Moreover, it did not restrict the use of dental amalgams in any of the susceptible subpopulations that were identified by the 2010 Scientific Advisory Panel. The response focused on incorrectly criticizing the science that was presented in the petition, incorrectly and incompletely citing scientific studies to support FDA’s stance and showed little knowledge of the importance of risk assessment.
In fact, on page 1, Ms. Kux stated, “A central question in assessing the risk of dental amalgam is whether the levels of mercury vapor released from dental amalgam are harmful or are associated with adverse health effects and, if so, to what extent.” (See Appendix VI, FDA Response and Appendix VII FDA Admissions) Whereas, it is known that elemental mercury, the kind of mercury ‘gassing off’ of amalgam fillings 24 hours a day, is a neurotoxin and therefore, the EPA and ASTDR have established RELs that are easily exceeded in individuals with amalgam fillings (discussed in depth later), and that is ‘The Central Question’ – Are American people with amalgam fillings exceeding those limits on a daily basis, which can add up to years of exposure to this neurotoxin? The weight of the evidence is heavy, as will be presented. However, the FDA’s expectation that prospective randomized controlled trials that would show definitive proof are necessary is ill-conceived, as such trials would be unethical and, these types of studies have not been funded by the federal government. The funding opportunities are not there*, even with the FDA’s repetitive statements in Leslie Kux’s response that “there-is limited to no clinical information available regarding longterm health outcomes” and “further study is needed”.
Leslie Kux, FDA, also states in the 2015 response, in regard to amalgam fillings: “It has a broad range of applicability in clinical situations, is easy to use, and is relatively insensitive to variations in handling technique and oral conditions. It also provides high strength, durability, and marginal integrity- features that may help prevent recurrent decay”. If ever they were, these statements are no longer true, a multitude of evidence exists clearly showing the superiority of composite fillings over amalgams. See Appendix II.
Leslie Kux, FDA, showcases the original Casa Pia Children’s study, one which has been severely criticized as ‘the’ study upon which the FDA bases its Final Rule on amalgam safety in children. See Appendix VIII for a summary of the criticisms and new findings related to the Casa Pia Study. She misrepresents the science, underplays the deficiencies in the science supporting the FDA stance and states nonsensical conclusions, such as this one when describing the Barreguard et al study of 2008: “In the New England trial,[10] groups of children had amalgam or composite restorations placed at ages 6-8 and were followed for 5 years. Results showed that, although microalbuminuria levels [a biomarker of renal glomerular injury] were higher in the amalgam treatment group, the levels of three other biomarkers of kidney injury were not different between the amalgam versus composite restoration groups”. Are we to just ignore that a biomarker of renal injury was elevated in children with amalgams because other biomarkers were not elevated?
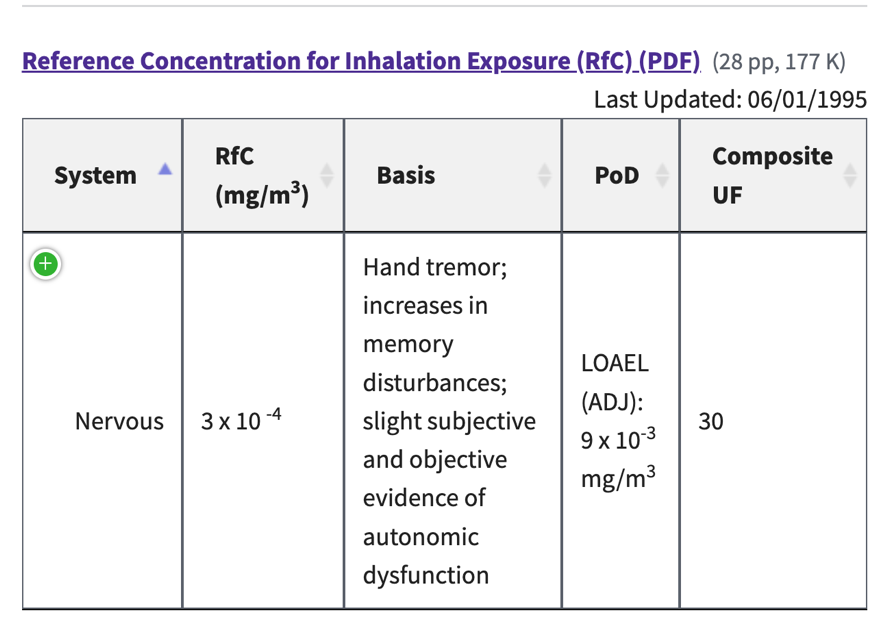 Leslie Kux, FDA, states repeatedly in the 2015 response that “FDA also believes that even though amalgam patients with numerous amalgam-filled surfaces could be exposed to daily doses of mercury vapor above the available RELs, this alone does not necessarily indicate that adverse health effects from dental amalgam will occur.” These types of statements clearly elucidate that Leslie Kux and the FDA choose to ignore the essence of why RELs are established, why they are important, and why they must be followed. For example, in the EPA’s Integrated Risk Information System (IRIS) under Mercury, elemental; CASRN 7439-97-6 the following information can be found, explaining why and how such limits are derived: ” The inhalation Reference Concentration (RfC) …. is based on the assumption that thresholds exist for certain toxic effects such as cellular necrosis. The inhalation RfC considers toxic effects for both the respiratory system (portal-of-entry) and for effects peripheral to the respiratory system (extra-respiratory effects). It is expressed in units of mg/m3. In general, the RfC is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily inhalation exposure of the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. Inhalation RfCs were derived according to the Interim Methods for Development of Inhalation Reference Doses (EPA/600/8-88/066F August 1989) and subsequently, according to Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (EPA/600/8-90/066F October 1994)”. This IRIS on mercury was derived and is supported by a number of scientific studies[11] – all of which, the FDA has chosen to ignore.
Leslie Kux, FDA, states repeatedly in the 2015 response that “FDA also believes that even though amalgam patients with numerous amalgam-filled surfaces could be exposed to daily doses of mercury vapor above the available RELs, this alone does not necessarily indicate that adverse health effects from dental amalgam will occur.” These types of statements clearly elucidate that Leslie Kux and the FDA choose to ignore the essence of why RELs are established, why they are important, and why they must be followed. For example, in the EPA’s Integrated Risk Information System (IRIS) under Mercury, elemental; CASRN 7439-97-6 the following information can be found, explaining why and how such limits are derived: ” The inhalation Reference Concentration (RfC) …. is based on the assumption that thresholds exist for certain toxic effects such as cellular necrosis. The inhalation RfC considers toxic effects for both the respiratory system (portal-of-entry) and for effects peripheral to the respiratory system (extra-respiratory effects). It is expressed in units of mg/m3. In general, the RfC is an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily inhalation exposure of the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime. Inhalation RfCs were derived according to the Interim Methods for Development of Inhalation Reference Doses (EPA/600/8-88/066F August 1989) and subsequently, according to Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (EPA/600/8-90/066F October 1994)”. This IRIS on mercury was derived and is supported by a number of scientific studies[11] – all of which, the FDA has chosen to ignore.
In May of 2019 the FDA solicited input from the American public on medical devices, including amalgam, to inform regulatory decision-making. device. Of the 278 comments that were received by the FDA on medical devices, 244 of those comments concerned amalgam. Not one of them condoned the use of amalgam and most asked for a ban or provided reasons why a ban should be instated. They spoke of personal experience with amalgam. They spoke of illness. They spoke of years of their lives, sometimes their entire lives, destroyed because of illness caused by amalgam fillings.[12]
In November of 2019 another FDA meeting was held, the goal of which was to advise the FDA on scientific issues related to metal implants.[13] An entire day of the two-day meeting was dedicated to the discussion of dental amalgam fillings. In advance of the meeting, a 186-page document, devoted to amalgam, was prepared by the FDA for themselves and the expert panel entitled Epidemiological Evidence on the Adverse Health Effects Reported in Relation to Mercury from Dental Amalgam: A systematic literature (2010 – Present). The document presented studies that had been conducted since the 2009 FDA meeting and conclusions drawn by the FDA regarding such. Interestingly, a study showing an alarming link between perinatal death and dental amalgam exposure during pregnancy was not in the document.[14] (See Appendix X, FDA Omissions for this and other omissions) Another study that was omitted from the document compared the health status of 600 dentists to a group of non-dentists, controlling for important variables. The comparison was done by accessing their pharmacy use. The study found that dentists took significantly more medications than non-dentists, for many diseases including neurological and cardiovascular disease. A full description of this and other epidemiological studies conducted since 2019 are included in Appendix XI.
In the Executive Summary of the 2019 report the FDA conclude “…the current evidence is insufficient to support a causal association between mercury from dental amalgam and reported adverse health effects. This is consistent with the assessments of other scientific organizations such as the recent SCENIHR report (2015, European Union) which concluded that dental amalgam does not pose a health risk for the general population…” This SCENIHR assessment, cited by FDA is no longer true (See Appendix XII). Thus, the FDA must consider and respect that the SCENIHR now recognize the dangers of mercury and amalgam fillings are banned across the entire European Union and many other countries (See Appendix XIII).
Four hundred-sixty-three public comments were received in advance of this November 2019 FDA meeting; many were submitted by scientists, many by mercury-toxicity sufferers. Individuals and members of special interest groups attended the meeting and spoke. Most of the comments regarding amalgams and all of the amalgam speakers, sans the ADA representative, made a plea for regulation to be placed on the use of amalgam. Regardless of the 186-page document, which made it clear that the FDA was not going to budge from their previous stance on amalgam, by the end of the meeting, most of the expert panel members agreed that mercury amalgam fillings have had their heyday. One panel member, Dr. Jason Connor stated, “If a product came on the market today and it was made with a material that is 50% highly toxic and we are predominantly going to be using it in disadvantaged populations, we wouldn’t be having a meeting. The FDA would not approve it.”
The general consensus by the panel of experts was to move forward with some form of regulation for amalgam. This was ignored by the FDA Chairman, Dr. Raj Rao. In fact, among several of his comments stating that we don’t have enough evidence to say amalgam isn’t safe (and this was challenged by panel members), he stated that “[maybe] the FDA announcements for mercury levels in fish could be revisited to be a more comprehensive announcement of the overall potential effects from mercury from fish, from dental amalgams and from the environment at large. That could be something to look into.” A link to the video-cast of the meeting is no longer available publicly but FDA surely has access to it in their archives. Dr. Rao’s statement can be found on Day 2, Hour 6:27.
Why would the FDA go to the trouble to hold this monumental meeting and invite prestigious experts to sit on the panel if they were going to remain true to their original stance? Perhaps, the FDA meeting was prompted by the third meeting of the Minamata Convention on Mercury, which was scheduled to be held less than two weeks after the FDA meeting. One purpose of the Minamata Convention Meeting was to consider whether the previously agreed-to world-wide amalgam phase down should be revised to a complete phase out.
The Minamata Convention meeting most certainly prompted the American Dental Association (ADA) commentary that was published just the month prior. The general gist of the ADA commentary, published in October 2019, is that it would be a very bad idea to ban the use of amalgam.[15] Among several reasons provided as to why a phase out would be “premature and counterproductive”, the authors state that “superior alternatives [to amalgam fillings] have not made it to the public sector”. That is a false statement (See Appendix II). The authors also imply that composites are too hard for dentists to place. If that is true, without being forced to, why are over 50% of all American dentists not using amalgam anymore? According to a survey conducted over 10 years 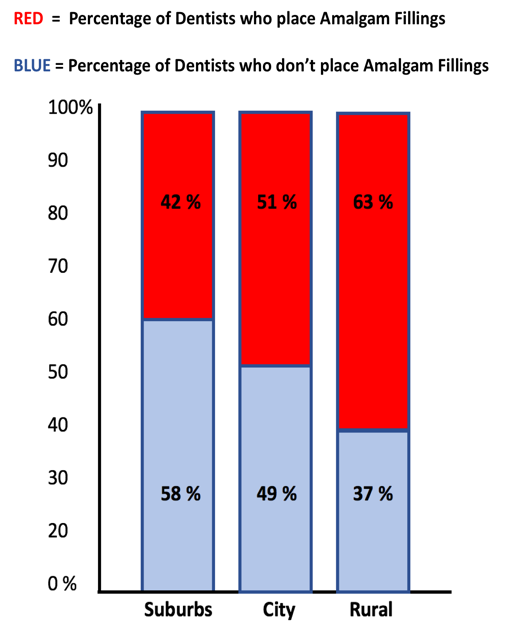 ago, and although it varies by state, over half of all dentists in the U.S. do not place amalgam fillings.[16] It also varies by locale such that rural area dentists place the most amalgam and suburban area dentists place the least. A more recent study confirmed the findings.[17] If approximately half of all dentists in the U.S. are NOT placing amalgams, which are the cheaper and easier to place alternative and result in greater profit for the dentist, what do they know that the other half choose to ignore? Should we assume that they have more skill than the 50% that are still using it? Are we to assume that European dentists are more skilled than American dentists? Because, dental amalgam is banned in all of E.U. and many additional countries (See Appendix XIII). Most likely, anyone reading this document, goes to a dentist who does not use amalgam. At the end of the day, don’t we want that for everyone?
ago, and although it varies by state, over half of all dentists in the U.S. do not place amalgam fillings.[16] It also varies by locale such that rural area dentists place the most amalgam and suburban area dentists place the least. A more recent study confirmed the findings.[17] If approximately half of all dentists in the U.S. are NOT placing amalgams, which are the cheaper and easier to place alternative and result in greater profit for the dentist, what do they know that the other half choose to ignore? Should we assume that they have more skill than the 50% that are still using it? Are we to assume that European dentists are more skilled than American dentists? Because, dental amalgam is banned in all of E.U. and many additional countries (See Appendix XIII). Most likely, anyone reading this document, goes to a dentist who does not use amalgam. At the end of the day, don’t we want that for everyone?
Finally, on September 24, 2020 the FDA posted ‘recommendations’ on its website that mercury amalgam restorative material not be placed in certain groups of people who may be at greater risk of potential adverse health effects caused by mercury exposure from amalgams. These groups include:
- Pregnant women and their developing fetuses;
- Women who are planning to become pregnant;
- Nursing women and their newborns and infants;
- Children, especially those younger than six years of age;
- People with pre-existing neurological disease;
- People with impaired kidney function; and,
- People with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.
Note that the described susceptible subpopulations are virtually identical to the subpopulations described by the 2010 Scientific Advisory Panel and very similar to the subpopulations for which my 2009 Petition sought protection. Note that Appendix XIV shows that 85% of US citizens, or 295,205,000 million people fall into these categories and are at risk, per the FDA, from amalgam fillings.
Following the entry of the FDA’s new position on amalgam fillings, the IAOMT and the ADA issued press releases reflecting their respective positions on the FDA’s current stance on amalgam. The IAOMT continued to call for eliminating the use of this material. The ADA stressed that there “was no new scientific evidence cited as part of the FDA recommendation.” While that may be true, the ADA doesn’t seem to understand the full history of FDA regulation of this material. As described above, the 2010 Scientific Advisory Panel identified the subpopulations in need of protection relying on science that was published before those hearings. There was no need to generate new science to justify the FDA’s change of position; it already existed. It remains to be known why in 2020, the FDA chose to embrace a ten-year-old Scientific Advisory Panel position.
Regardless of the history attesting to the evasion of their duty to protect US citizens, we are hopeful that the FDA will stand by its promise, restated by Ms. Kux “…that the agency continues to evaluate the literature on dental amalgam and any other new information it receives in light of the 2010 panel recommendations, and will take further action on dental amalgam as warranted”.
In addition to the science that was presented in the 2009 petition, that has previously been criticized by FDA through Leslie Kux’s response, we have included here in Appendix I over 150 recent studies clearly outlining mercury amalgam’s effects on various endpoints and in various diseases. Some of the newer epidemiology studies that are listed in the table are described in more detail in Appendix XI, demonstrating amalgam-related retinal neurotoxicity, perinatal death related to amalgam filling exposure during pregnancy, elevated neuropsychiatric and cardiovascular disorders in dentists, and associations between amalgam and incidence rates of asthma and arthritis.
We have also included Appendix XV which describes DNA/RNA studies not included in the FDA’s 2019 report. It is well-known that alterations in DNA/RNA can lead to genetic disorders, developmental problems and increase risk of cancer and other disease. Since 2019, research has accrued in this arena.
D. Statement of Grounds:
On July 28, 2009, FDA announced that it was classifying dental amalgam for the first time in Class II without requiring any significant special controls. FDA’s Final Rule on this issue was published on August 4, 2009. FDA also published an Addendum in support of its Final Rule, where FDA explained its attempts to address the recommendations of the Joint Panels that convened in September 2006 and rejected the conclusions in the FDA White Paper on amalgam fillings.
To protect the American public, under 21 U.S.C. § 360f, mercury amalgam dental fillings must be banned. Unlike other mercury-based medical products that have been removed, amalgam remains on the market under FDA’s outdated and inadequate “Class II Special Controls Guidance.”
The FDA claims the guidance ensures safety and effectiveness, yet it dismisses known health risks and relies on obsolete data. The document lacks transparency—making unreferenced claims about mercury exposure in children and breastfeeding infants. Most importantly, the FDA has used this Special Controls document to misinterpret the ‘learned intermediary doctrine’.
As an example of the outdatedness of the Special Controls guidance, FDA cites the HHS 1993 Scientific review to support the statement “Dental amalgam has been demonstrated to be an effective restorative material that has benefits in terms of strength, marginal integrity, suitability for large occlusal surfaces, and durability.” If there wasn’t then, over thirty years later, there is more than sufficient evidence to refute this claim (See Appendix II).
To provide an example of the vagueness of the Special Controls guidance, the following statement is provided to guide industry on information to include on amalgam labeling: “Taking into account factors such as the number and size of teeth and respiratory volumes and rates, FDA estimates that the estimated daily dose of mercury in children under age six with dental amalgams is lower than the estimated daily adult dose. The exposures to children would therefore be lower than the protective levels of exposure identified by ATSDR and EPA”. The FDA provides this statement without including references as to how the calculations were made, and of which, as we will show below, the FDA have not provided such risk assessments.
The FDA also state that exceeding the levels of exposure for mercury that would provide protection established by the ATSDR and EPA “…does not necessarily mean that any adverse effects will occur”. It is difficult to determine if this is just vague or if it is doublespeak.
To provide an example of the FDA’s denial of adverse health effects of amalgam, the FDA states “In addition, the estimated concentration of mercury in breast milk attributable to dental amalgam is an order of magnitude below the EPA protective reference dose for oral exposure to inorganic mercury. FDA has concluded that the existing data support a finding that infants are not at risk for adverse health effects from the breast milk of women exposed to mercury vapors from dental amalgam.” However, there is clear evidence that infants are at risk (see Appendix I, Perinatal, Pregnancy and Reproductive categories). Not only does the FDA deny the known risks of dental amalgam to the infants of women who breastfeed, but it is provided without providing any reference as to how the FDA came to this conclusion – in other words, they have not conducted this risk assessment.
FDA further undermines safety by misapplying the learned intermediary doctrine.[18] In denying our 2009 petition and again in response to Docket Nos.: FDA-2015-P-3876, FDA-2016-P-1303, FDA-2016-P-3674, and FDA-2017-P-2233, submitted by Charles G. Brown (see Appendices VI and XVI), the agency stated dentists need not inform patients about amalgam risks because they act as learned intermediaries. This contradicts the doctrine, which obligates providers to inform patients of known risks. FDA’s pervasive approach, spanning at least 7 years (2009-2015), shifts liability to dentists and shields industry.
Notably, the guidance recommends industry provide labeling such as: WARNING: CONTAINS MERCURY. May be harmful if vapors are inhaled. Yet FDA says patients need not be informed—despite being the victims of 24 hour a day exposure to mercury vapor. This failure to require informed consent violates public trust and patient safety. Therefore, the current Special Controls are not adequate, and mercury amalgam must be banned.
A secondary alternative is that they should be immediately placed in Class III [12 U.S.C. § 360c]. Mercurial wound disinfectants, diuretics, thermometers, vaccines, batteries, veterinary substances have been eliminated for safety reasons and yet mercury amalgams are still being placed in mouths where it invades the body, particularly the brain, liver and kidneys. There is no magic that makes dental mercury safer than those obsolete products of the past. In this era when the public is advised to be concerned about mercury exposure through fish and other food consumption, the FDA should ban mercury fillings as the predominant source of mercury exposure in the general population.
There are several obvious flaws in the FDA’s Final Rule, as follows:
- FDA Final Rule on the classification of dental amalgam is based on a superficial and inadequate review of the literature.
- The estimated mercury vapor exposure from dental amalgam is incomplete, ill-composed, ill-conceived, indefensible, and inaccurate.
- An effective and defensible risk assessment for mercury vapor complies with EPA (2004, 1998, 1994) and the National Academy of Sciences (NAC, 2008).
- FDA fails to utilize a methodical analysis of the ”weight of evidence” of the toxicological literature.
- FDA offers no detailed quantitative analysis of its toxicological database leading to the determination of a defensible regulatory reference exposure level.
- FDA fails to utilize a methodical, transparent, and defensible quantification of exposure for comparison to that reference exposure level.
- FDA makes no defensible attempt to compare the full range of mercury exposures across the entire amalgam-bearing U.S. population to regulatory reference exposure levels designed and intended to protect the general population.
- FDA only considers exposures attributed to a maximum of ten filled surfaces, and only in adults, but incorrectly assumes this also applies to children six years and older.
- The FDA ignores children younger than six years, but children as young as three years receive amalgam fillings.
- The FDA ignores persons with more than ten amalgam surfaces, but adults often have up to twenty-five (and possibly more) amalgam-filled surfaces on their teeth.
- The FDA makes no attempt to determine the number or percentage of Americans excluded from its risk assessment.
- The FDA omits to quantify the full range of mercury exposure across the entire population, in all relevant age groups.
- The FDA omits to quantify the proportion of the amalgam-bearing population that exceeds the Environmental Protection Agency’s (EPA) references concentration (RfC) and the Agency for Toxic Substances and Disease Registry’s(ATSDR) minimum risk level (MRL), the two reference exposure levels that purportedly provide health protection to the non-occupationally exposed general population.
- The FDA omits to quantify the exposure in children less than six years of age, an age group considered the most vulnerable to exposure and adverse effects and a population group that receives amalgam fillings.
- Many of the FDA calculations in the final rule are in error, in part due to improvident reliance on outdated or non-authoritative sources of information.
- FDA utilizes unreliable values for its assumed inhalation rate; FDA relies on EPA’s RfC but inexplicably fails to recognize EPA (1997; 2008) as the most nationally and internationally authoritative information source on human inhalation rates.
- The RfC-associated dose and MRL-associated dose is improperly extrapolated to apply to children. These doses should only be derived for adults, the age group studied in the occupational studies upon which the RfC and MRL are based.
- FDA fails to adjust inhaled dose for the 80% absorption of mercury vapor in the lungs.
- FDA fails to standardize the internal doses associated with the RfC and MRL (and those from amalgam) to body weight due to the great disparity in body weights in the different age groups being considered.
- Contrary to the FDA’s statement, the WHO Environmental Health Criteria 118 (WHO 1991) did not “[find] that values generally in the range of 1-5 µglday were estimated in the US. adult population”. Rather, WHO (1991) concluded that “[e]stimated average daily intake and retention” from dental amalgam was 3.8-21 (3-17) µg/day (values in brackets representing retained (absorbed) dose (WHO, 1991, Table 2).
- Contrary to FDA’s assertion, the WHO (2003) did not conclude that “[t]he highest estimate that WHO reports is a dose of 12 µg/day, for middle-aged individuals with approximately 30 amalgam surfaces (Ref. 22).” In the Executive Summary of the document (WHO 2003), WHO clearly states “dental amalgam constitutes a potentially significant source of exposure to elemental mercury, with estimates of daily intake from amalgam restorations ranging from 1 to 27 µg/day.”
- Keeping in mind that teeth have up to 5 surfaces; each surface covering constitutes ‘a filling’. Thus, a single tooth can have up to 5 amalgam fillings.
Based on FDA’s method of estimating mercury exposure from dental amalgam, and assuming that the RfC is derived correctly, the number of fillings necessary to exceed the RfC are:
- Child 3-6 yrs – 2 fillings
- Child 6-11 yrs – 2 fillings
- Teen 12-19 yr- 3 fillings
- Adults – 7 fillings
Based on FDA’s method of estimating mercury exposure from amalgam, and assuming the MRL is derived correctly, the number of fillings that result in exceeding the MRL are:
- Child 3-6 yrs – 2 fillings
- Child 6-11 yrs – 2 fillings
- Teen 12-19 yr- 4 fillings
- Adults – 5 fillings
The FDA has inadequately quantified mercury exposure in, or totally omitted to consider, the following Americans:
- 428,000 American toddlers aged three and four years that possess amalgam filled teeth, and 260,000 of these toddlers that would exceed the MRL-equivalent dose of mercury from their amalgam fillings, and 61,000 toddlers who would exceed the RfC-equivalent dose for mercury.
- 11,386,000 American children between the ages of five and eleven who may possess amalgam filled teeth, bearing from one to sixteen amalgam-filled teeth. Of these children, 5,909,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 3,205,000 would exceed the RfC-equivalent dose for mercury vapor.
- 19,856,000 American teens between the age of twelve and nineteen who may possess between one and twenty-two filled teeth, for whom the FDA considered it unnecessary to quantify their precise mercury exposure from dental amalgam. Of these teens, 6,378,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 2,965,000 would exceed the RfC-equivalent dose for mercury. Also in this age group, nearly three million would have more than ten filled teeth; in excess of the number of amalgam-filled teeth (and their associated dose and potential health effects) even considered by the FDA in their Final Rule.
- Up to 118 million adult Americans who may possess between one and twenty-five teeth containing amalgam. Of these, 43,550,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 21,682,000 would exceed the RfC equivalent dose for mercury. Also in this age group, nearly 44 million would have more than ten filled teeth; more than the number of amalgam-filled teeth (and their associated dose and potential health effects) even considered by the FDA in their Final Rule.
- In all, between the young age groups ignored in the FDA Final Rule, and those with more than ten filled teeth, also ignored in the FDA Final Rule, some 48 million Americans are omitted from consideration by the FDA.
The FDA failed to recognize or rectify the inadequacy and non-valid nature of the EPA RfC or the ATSDR MRL:
- The EPA categorizes mercury vapor as a neurotoxin but the RfC has not yet been revised and updated to comply with EPA’s (1998) guidance on the assessment of neurotoxins nor the guidance provided by the National Academy of Sciences (NAS 2008).
- The EPA acknowledged as early as 2002 that significant new literature was available on the toxicity of mercury vapor; FDA cannot properly cite EPA’s lack of action to revise the RfC and address the new literature as ”evidence” of the lack of new and significant studies.
- The reviews by EPA (1995) and the ATSDR (1999) are not recent, as indicated by FDA; the EPA RfC cites no literature later than 1995, now some thirty years out-of-date. Interestingly, a handful of newer citations have been added to the ATSDR Toxicological Profile on Mercury but only a few, and only those that support amalgams as being a safe dental material. A table is included in the latest information, showing several funded studies that aim to study the safety of mercury and/or amalgams. None of these funded studies appear to be active.
- FDA claims to have reviewed relevant literature up to July 2009, but it failed to locate Health Canada (2006), Richardson et al. (2009), Ratcliffe et al. (1996), among many other relevant studies and reports, discussed below.
- The FDA failed to recognize that studies of workers at chloralkali plants, where concomitant exposure to mercury vapor and chlorine gas occurs, are invalid for establishing reference exposures levels for non-occupational exposure to Hgº.
- Mercury has been identified in a large number of peer reviewed studies as being a likely cause of the more prevalent neurological disorders such as Alzheimer’s Disease, severe autism, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Parkinson’s Disease (PD). Mercury also causes hearing loss, periodontal disease, kidney dysfunction, and allergy.
- FDA failed to prepare an environmental impact study, or at least an environmental assessment, in violation of the National Environmental Protection Act.
1. Introduction
The FDA final rule on amalgam is based on a superficial review of the literature on the health effects of mercury vapor, and estimates of mercury vapor exposure from dental amalgam, both of which are incomplete, ill-composed, ill-conceived and inaccurate. Although purporting to be a ‘risk assessment’, the documentation is nothing of the sort. An effective and defensible risk assessment complies with the standards of practice endorsed and espoused by the professional risk assessment community. Those standards of practice have been well presented and expressly documented by the US EPA (2004, 1998, 1994) and by the US National Academy of Sciences (US NAC, 2008). Those standards of practice demand: 1) a methodical analysis of the ‘weight of evidence’ of the toxicological literature; 2) a detailed quantitative analysis of that toxicological database towards the determination of a defensible regulatory reference exposure level; and 3) a methodical, transparent and defensible quantification of exposure for comparison to that reference exposure level. All three of these critical steps are missing from the FDA final rule.
2. What is a defensible regulatory risk assessment?
An effective and defensible risk assessment of dental amalgam requires a detailed quantitative analysis of the exposure to mercury vapor in the general population. However, the FDA only alludes to average or typical exposure levels, citing dated (predating 1993) reviews which they themselves only cite other yet older reviews.
A typical, defensible regulatory risk assessment for chemical exposure would quantify that exposure across the entire general population, and particularly in the ‘reasonably maximally exposed’ portion of the US population, not just some undefined average or typical person. To achieve this, data on the range (minimum to maximum) of that chemical exposure across all members of the general population is required. Unfortunately, with respect to mercury vapor exposure from dental amalgam, the FDA fails to quantify exposure in those members of the US population who are maximally exposed- those with up to twenty-five amalgam-filled surfaces on their teeth. The FDA only considers those with up to ten amalgam fillings.
Further, a defensible risk assessment includes all segments of the US population. However, the FDA never attempted to quantify mercury exposure in children under six years of age, despite knowledge that children as young as 3 years of age do receive amalgam fillings and, as a result, are exposed to mercury vapor from this source. The significance of this oversight is compounded by the fact that risk assessment guidance for neurotoxic agents such as mercury vapor (see USEPA 1998) specifically stipulates the importance of considering infants and young children in whom neurotoxicity will be pronounced due to the susceptibility of the growing and developing brain to the effects of neurotoxins.
To demonstrate that such an exposure assessment is possible and feasible, the Canadian government, in its risk assessment of dental amalgam (Health Canada, 1995) was open and transparent about the prevalence of mercury fillings in the Canadian population, with adults having up to 25 filled surfaces on their teeth and children as young as 3 years of age having amalgam fillings. Health Canada was also explicit in the methods used to estimate exposures, to the point of providing estimates of mercury vapor exposure per filled surface, for each of five separate age groups (i.e., toddlers, children, teens, adults and seniors). Health Canada neither omitted to determine exposure in persons with more than 10 fillings, nor omitted to consider children less than 6 years of age. Both such considerations were omitted by the FDA in their final rule.
3. What is an appropriate risk characterization? (What reference levels should exposures be compared to?)
Although FDA appears to agree that reference air concentrations derived for the protection of the non-occupationally exposed, general population should be employed for the assessment of potential risks posed by amalgam (From FDA Final Rule: “These reference values… are considered to represent chronic or lifetime inhalation exposures that are free from adverse health outcomes and protective of human health for all individuals, including potentially sensitive populations such as children prenatally or postnatally exposed to mercury vapour.”), the only comparisons the FDA presents relate to effects and exposure levels reported in occupational studies of adults. There was no attempt to accurately quantify exposure to mercury vapor arising from the use of dental amalgam in the general US population, nor to compare those exposure levels to the reference air concentration (RfC) published by the US EPA (EPA, 1995) or the minimal risk level (MRL) published by the ATSDR (1999), both reference levels established for the protection of that non-occupationally exposed U.S. general population. Health Canada (1995), on the other hand, directly compared mercury vapor exposure from dental amalgam to such a reference exposure level specifically derived for the protection of the general population.[19]
4. How detailed and precise should exposure assessments be?
The lack of precision offered by FDA with respect to the average exposure to mercury from dental amalgam, not to mention their total failure to dependably quantify the range of exposure including those maximally exposed and those younger than six years of age, is disconcerting. The FDA has failed to adequately quantify:
• the full range of exposure across the entire population, in all relevant age groups;
• the proportion of the amalgam-bearing population that exceed the US EPA RfC and the ATSDR MRL, the two reference exposure levels identified by the FDA as providing health protection to the non-occupationally exposed general population;
- the exposure in children less than 6 years of age, an age group considered the most vulnerable to exposure and effects and a population group that receives amalgam fillings.
5. Doses Associated with the EPA RfC and the ATSDR MRL versus FDA’s Ill-Defined Exposure Levels for Adults and Children Six Years of Age and Older
a. Internal doses associated with the RfC and MRL
The FDA attempts to convert the RfC and MRL to an absorbed dose in their Final Rule, incorrectly estimating the following internal doses:
| Age group | RfC-associated intake (µgs/day) | MRL-associated intake (µgs/day) |
| Adults | 4.9 | 3.2 |
| 5 years old Children | 2.3 | 1.5 |
| 1 year old Infants | 1.7 | 1.2 |
In calculating these absorbed doses, the FDA makes five key errors.
- it uses unreliable values for inhalation rates;
- it fails to adjust the inhaled doses for the 80% absorption of mercury vapor in the lungs, an absorption rate acknowledged elsewhere in FDA’s Final Rule;
- it fails to standardize the internal doses associated with the RfC and MRL (and those from amalgam) with various body weights to account for the great weight disparities found in the different age groups under consideration.
- the RfC-associated dose and MRL-associated dose is derived for adults only, the age group studied in the occupational studies upon which the RfC and MRL are based; and
- the RfC-associated dose and MRL-associated dose is derived as if all surfaces of a tooth are the same size, and therefore, all amalgam fillings are the same size. Neither of which is true. Teeth vary significantly in size (molar vs incisor) and among individuals (adult male vs a 3 year old), as does the extent of the decay, as does the amount of amalgam filling required.
b. Inhalation and Absorption Rates
Rather than accessing the most nationally and internationally authoritative data and information on inhalation rate that was compiled and thoroughly analyzed by the US EPA (1997; 2008) — the FDA chose to estimate inhalation rates based on only two citations. US EPA’s Exposure Factors Handbook (EPA 1997) reviews twenty-one key and dependable studies to determine that the adult inhalation rate is 13.25 m3/day for males and females combined. This is significantly less than FDA’s undependable estimate of 16.2 m3/day.
The FDA acknowledges on page 8 of its Final Rule that the inhaled absorption rate for mercury vapor is 80%, yet it fails to apply this factor to its calculations in deriving the absorbed doses based on the RfC and MRL. Instead, FDA assumes 100% absorption of the inhaled mercury vapor. This error incorrectly pushes the permissible dose higher than it should be.
c. Standardization to Account for Body Weight
In order to conduct any form of comparison of the FDA’s assumed mercury vapor dose (1 to 5 µgs per seven to ten fillings) to the EPA RfC or ATSDR MRL (0.3 µgs/m3 and 0.2 µgs/m3, respectively) it is necessary to convert both the exposure estimates and the reference exposure levels to the same units. To do this, both must be converted to absorbed, weight-standardized doses in units of µgs/kg body weight/day.
The internal dose associated with the EPA RfC for mercury vapor (0.3 µgs/m3) can be determined by consideration of inhalation rate and body weight in adults, the population group investigated in the occupational epidemiology study upon which the RfC was based, and adjusting for 80% absorption. According to the US EPA, adult average inhalation rate is 13.25 m3/day (EPA, 1997; average of males and females) and average adult body weight is 71.8 kg (EPA 1997; average of males and females). Assuming that 80% of inhaled mercury vapor is absorbed (as assumed by the FDA in their Final Rule), the internal RfC-associated reference dose is: (0.3 µgs/m3 x 13.25 m3/day X 80%)/71.8 kg= 0.044 µgs/kg body weight/day. For the MRL of 0.2 µgs/m3, the equivalent internal MRL-associated reference dose is similarly derived as 0.03µgs/kg bw/day.
6. Mercury Exposure from Dental Amalgam
The FDA cites an ill-defined and unsubstantiated estimate of absorbed mercury exposure from dental amalgam of 1 to 5 µgs/day that supposedly relates to the presence of between 7 and 10 amalgam fillings. This conclusion is attributed to a report by the Public Health Service published in 1993 (PHS, 1993). This cited report did not contain or conduct a detailed quantification of mercury exposure but based its estimates on the review of other yet older reports. In fact, PHS (1993) acknowledged that estimates of mercury exposure from amalgam span 1 µg/day to 29 µgs/day (see PHS, 1993, Appendix III), with higher estimates appropriately acknowledged for the sizable population of persons who have more than ten amalgam fillings.
Contrary to the FDA’s statement, the WHO Environmental Health Criteria 118 (WHO 1991) did not “[find] that values generally in the range of 1-5 µg/day were estimated in the US. adult population”. Rather, WHO (1991) concluded that “[e]stimated average daily intake and retention” from dental amalgam was 3.8-21 (3-17) µgs/day (values in brackets representing retained (absorbed) dose (WHO, 1991, Table 2). Contrary to FDA’s assertion, the WHO (2003) did not conclude that “[t]he highest estimate that WHO reports was a dose of 12 µgs/day, for middle-aged individuals with approximately 30 amalgam surfaces (Ref 22)”. In the Executive Summary of this document (WHO 2003), WHO clearly states “Dental amalgam constitutes a potentially significant source of exposure to elemental mercury, with estimates of daily intake from amalgam restorations ranging from 1 to 27 µgs /day.”
7. Comparing Mercury Exposure from Amalgam to the Reference Exposure Levels for the General Population
In order to conduct any form of comparison of the FDA’s assumed mercury vapor dose (1 to 5 µgs per 7 to 10 fillings) to the EPA RfC or ATSDR MRL (0.3 µgs /m3 and 0.2 µgs/m3, respectively) it is necessary to convert both the exposure estimate and the reference exposure level to the same units. To do this, both must be converted to absorbed, weight-standardized doses in units of µgs /kg body weight/day.
If we assume, arguendo, that ten amalgam fillings deliver a daily dose of mercury of 5 µgs/day as an absorbed dose (per the FDA Final Rule), then one filling delivers an absorbed dose of 0.5 µgs/day. When standardized to body weight, as is routine for toxicological reference exposure levels and exposure assessments, this daily dose represents differing doses for different age groups with differing average body weights. Using data on body weights of different age groups provided by the EPA (2008), the weight-standardized doses associated with that 0.5 µgs/day dose are:
| Age group | Body weight | Weight-standardized dose per filling (after FDA) |
Number of fillings to exceed EPA RfC |
Number of fillings to exceed ATSDR MRL |
| 3-6 year
olds |
18.6 kg | 0.027 µgs/kg bw/day | 2 | 2 |
| 6-11 year
olds |
31.8 kg | 0.016 µgs/kg bw/day | 3 | 2 |
| 12-19 yrs | 56.4 kg | 0.009 µgs /kg bw/day | 5 | 4 |
| Adults : ≥20 yrs | 71.8 kg | 0.007 µgs /kg bw/day | 7 | 5 |
Assuming FDA is correct in its estimate of dose associated with ten amalgam fillings, this table clearly demonstrates the following conclusions:
- Weight-standardized dose increases as body weight (and age) decreases;
- The weight-standardized dose to young children (aged 3-6 years) is almost four times greater than the weight-standardized dose to adults, due entirely to the difference in body weights between these age groups;
- Young children who have two or more amalgam fillings exceed the weight-standardized absorbed dose associated with the EPA RfC and ATSDR MRL;
- Adults with seven or more amalgam-filled teeth will exceed the RfC and with five or more amalgam fillings will exceed the MRL;
- All age groups will exceed the doses associated with U.S. regulatory reference air concentrations with less than the average of seven to ten fillings assumed by the FDA to be ‘safe.’
We have no doubt that FDA has the resources and expertise to properly assess the risks associated with dental amalgam. Sadly, FDA’s clear priority is to defend at all costs the continued use of mercury in dentistry — even at the expense of the public health. It is not surprising, therefore, that FDA declined to validly and defensibly compare its estimate of the average or typical mercury vapor exposure to the very reference exposure levels it represents to be safe for the general population.
At this time, another risk assessment has been conducted. Using similar techniques to Richardson et al, a more recent and more accurate risk assessment has recently been undertaken by Geier and Geier (2022) to meet several goals:
- Quantify daily Hg vapor exposure;
- Determine how demographic covariates such as gender, age, race, country of birth, and socioeconomic status impact mercury vapor exposure;
- Determine the number of adults receiving daily mercury vapor doses in excess of various governmental safety limits;
- Determine how demographic covariates such as gender, age, race, country of birth, and socioeconomic status impact the number of adults receiving mercury vapor doses in excess of various governmental mercury vapor safety limits; and
- Determine the mean number of amalgam surfaces allowable for the average adult’s mercury levels to fall within the various governmental Hg vapor safety limits.
This study provides the first nationwide insights into the direct contribution of amalgams on mercury vapor exposure among American adults. An adult population of 158,274,824 weighted-persons ranging in age from 21-66 years was examined. Demographics, oral health examinations, urinary mercury amounts, measured bodyweight, and measured urine flow rates were extracted for all subjects from the 2015–2018 NHANES database (See Appendix XVII for full details of this study).[20]
The results listed in the table below show that 10.4% of US adults are exposed to mercury from amalgam fillings in excess of the EPA safety limit and 21.4% exceed the ATSDR limit. According to the research of Richardson et al 2011, featured at the FDA 2010 amalgam safety meeting, as discussed above, 45.7% of US adults have mercury levels that are in excess of the safety limit recommended.
| Mercury vapor safety limits | Number of persons (158,274,824) |
| US environmental protection agency (0.048 μg of Hg/Kg/Day) | 10.4% (16,419,510) |
| US Agency for toxic substances and disease registry (0.032 μg of Hg/Kg/Day) | 21.4% (33,875,805) |
| Health Canada (0.011 μg of Hg/Kg/Day) | 43.9% (66,448,434) |
| Richardson et al. (0.010 μg of Hg/Kg/Day) | 45.7% (72,257,809) |
| California’s environmental protection agency (0.005 μg of Hg/Kg/Day) | 54.3% (85,876,060) |
8. Assessing the Percentage of the Population Receiving Doses of Mercury that Exceed the RfC and the MRL
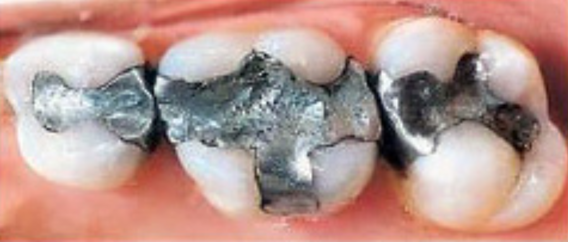 As previously stated, FDA states that mercury exposure from amalgam ranges between 1 and 5 µgs/day. However, that exposure level represents only the average exposure in adults, associated with possessing an average of seven to ten amalgam-filled teeth, while some adults have as many as 25 amalgam fillings. The image to the right shows two surfaces are covered by amalgam in the left-most tooth, four surfaces are covered in the middle tooth and two surfaces are covered by the right-most tooth. The FDA further assumes that this range of exposure occurs (and is safe) in children six years of age and older, as well as in adults. Given that the FDA final rule acknowledges that amalgam can be the single greatest source of exposure to mercury vapor in the U.S. population, it is astonishing that the FDA did not undertake a more quantitative and definitive analysis of exposure to mercury from amalgam, especially considering the billions of fillings placed in millions (10s to 100s) of Americans (statistics as described by FDA).
As previously stated, FDA states that mercury exposure from amalgam ranges between 1 and 5 µgs/day. However, that exposure level represents only the average exposure in adults, associated with possessing an average of seven to ten amalgam-filled teeth, while some adults have as many as 25 amalgam fillings. The image to the right shows two surfaces are covered by amalgam in the left-most tooth, four surfaces are covered in the middle tooth and two surfaces are covered by the right-most tooth. The FDA further assumes that this range of exposure occurs (and is safe) in children six years of age and older, as well as in adults. Given that the FDA final rule acknowledges that amalgam can be the single greatest source of exposure to mercury vapor in the U.S. population, it is astonishing that the FDA did not undertake a more quantitative and definitive analysis of exposure to mercury from amalgam, especially considering the billions of fillings placed in millions (10s to 100s) of Americans (statistics as described by FDA).
The other questions that FDA should have answered are:
- Just how many American adults with amalgam fillings are receiving a dose greater than either the EPA RfC or the ATSDR MRL?
- Just how many American children under six years of age with amalgam fillings are receiving a dose greater than either the EPA RfC or the ATSDR MRL?
These questions are answered below.
National Institute of Dental and Craniofacial Research (NIDCR) publishes data collected by NHANES on the average number of filled teeth in the American population (see, e.g., https://www.nidcr.nih.gov/research/data-statistics/dental-caries/adolescents NIDCR possesses the data to permit an accurate accounting of the number of persons with filled teeth in the U.S. population. These data would permit an accurate determination of mercury exposure across the full range of numbers of filled teeth in the U.S. population. It is unfortunate that the FDA did not avail itself of that data.
Given the comparability of living standards between Canada and the US, we will apply available Canadian data for these derivations here, as they will be comparable to the dental care/dental health status in the U.S. population. Based on data available from Health Canada (HC, 1995) on the proportion of various age groups bearing amalgam fillings, and 2009 US population census projections from the US Census Bureau (http://www.census.gov/popest/national/asrh/2008-nat-res.html) the following number of Americans with amalgam fillings are evident:
a. Up to 5.1% of American children aged 3 and 4 years of age may possess amalgam filled teeth, representing 428,000 American toddlers for whom the FDA considered it unnecessary to quantify their mercury exposure from dental amalgam. Of these toddlers, 260,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 61,000 would exceed the RfC-equivalent dose for mercury.
b. Up to 40.4% of American children between the ages of 5 and 11 may possess amalgam-filled teeth, bearing from one to sixteen amalgam-filled teeth, representing 11,386,000 American children for whom the FDA considered it unnecessary to quantify their precise mercury exposure from dental amalgam. Of these children, 5,909,000 would exceed the MRLequivalent dose of mercury from their amalgam fillings, while 3,205,000 would exceed the RfCequivalent dose for mercury.
c. Up to 59.3% of American teens between the age of 12 and 19 may possess between one and twenty-two filled teeth, representing 19,856,000 American teens for whom the FDA considered it unnecessary to quantify their precise mercury exposure from dental amalgam. Of these teens, 6,378,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 2,965,000 would exceed the RfC-equivalent dose for mercury. Also in this age group, 9% (nearly 3 million American teens) have more than 10 filled teeth; in excess of the number of amalgam-filled teeth (and their associated dose and potential health effects) even considered by the FDA in their Final Rule.
d. Up to 52.8% of the adult American population may possess between one and twenty-five filled surfaces on their teeth, representing more than 118 million Americans for whom the FDA considered it unnecessary to quantify their precise mercury exposure from dental amalgam. Of these, 43,550,000 would exceed the MRL-equivalent dose of mercury from their amalgam fillings, while 21,682,000 would exceed the RfC-equivalent dose for mercury. Also in this age group, 19.5% (nearly 44 million Americans) have more than 10 filled teeth; in excess of the number of amalgam-filled teeth (and their associated dose and potential health effects) even considered by the FDA in their Final Rule.
e. In all, between the young age groups ignored in the FDA Final Rule, and those with more than ten filled teeth, also ignored in the FDA final Rule, some 48 million Americans are receiving doses of mercury solely derived from their mercury fillings that exceed the MRL and the RfC. FDA should be especially concerned about these conclusions in view of the additional environmental exposure to mercury that is occurring in this country. Laks reports that the total exposure of the U.S. population to mercury is on the rise. “This study is the first to report that there is a rise in the mean blood iodine-mercury (I-Hg) (defined as “blood inorganic mercury”) detection and I-Hg concentration within the US population over time.” Laks also reports that his study “indicates that iodine/mercury deposition within the human body is significantly associated with biomarkers for the main targets of chronic mercury exposure, deposition and effect: the liver, immune system and pituitary. These correlations between chronic mercury exposure, I-Hg deposition, and biochemical profile markers for the targets of I-Hg deposition confirm strong links between exposure and associated disease.” FDA’s Final Rule does not consider this documented additional mercury derived from environmental (non-amalgam) sources and then compare that total mercury burden to the RfC and the MRL. Clearly, FDA’s analysis fails to offer a reasonable assurance of safety for a substantial portion of the U.S. population.[21]
9. Are the RfC and the MRL for Mercury Vapor Based on Current Knowledge?
a. The RfC and MRL are Outdated
In this section (9) of the FDA paper, there are incomplete references to published papers identified only by author and year. Each of these papers is discussed in Richardson, et al., (2009).[22]
The FDA incorrectly states that: “[the RfC and the MRL] are considered to represent chronic or lifetime inhalation exposures that are free from adverse health outcomes and protective of human health for all individuals, including potentially sensitive populations such as children prenatally or postnatally exposed to mercury vapour.” Castorina and Woodruff (2003)[23] clearly demonstrate that: “Although noncancer outcomes may in some instances be reversible and considered less severe than cancer, our findings call into question the assumption that established RID and RfC values represent negligibly small risk levels. ”
The EPA recognizes that mercury vapor is a neurotoxin. As such, the toxicological assessment by EPA of mercury and derivation of a suitable reference air concentration (RfC) must comply with EPA’s (1998) guidance on the assessment of neurotoxins. The publication of that EPA guidance occurred three years after the publication of EPA’s RfC for mercury vapor, thus indicating that this RfC is out of compliance with EPA’s own policies and procedures for the assessment of neurotoxins. It is apparent, therefore, that this RfC is out of date and will eventually be (must be) updated to accurately reflect both the latest literature on mercury vapor toxicity and EPA’s own neurotoxin risk assessment guidance.
The FDA incorrectly cites the EPA documentation associated with the out-of-date EPA RfC. The FDA allege that a 2002 contractor’s report (screening assessment), prepared for the US EPA on toxicological studies of mercury vapor published between approximately 1995 and 2002, is evidence that the EPA found no new data or information warranting revision of the EPA RfC
“A screening-level review conducted by an EPA contractor of the more recent toxicology literature pertinent to the RfC for Mercury, elemental conducted in September 2002 identified one or more significant new studies” [emphasis added] (see statement on “Screening-Level Literature Review Findings”, Section I.B.6, of the EPA IRIS listing on elemental mercury (http://www.epa.gov/ncea/iris/subst/0370.htm)).
Although it is apparent that the EPA has yet to consider these new studies with respect to revising or updating its RfC, this inaction by EPA cannot be properly cited by the FDA as ‘evidence’ of a dearth of new and relevant studies. The EPA RfC was first published in 1995 (see https://iris.epa.gov/ChemicalLanding/&substance_nmbr=370 and has not been updated for new toxicological studies since that time. In fact, contrary to the supposition of the FDA, the most recent study cited by the US EPA in support of its RfC is 1995.
FDA states that the EPA (1995) and ATSDR (1999) constitute ‘recent’ reviews of the toxicological literature on mercury vapor. This is incorrect. As previously mentioned, the EPA RfC cites no literature later than 1995, now some 30 years out-of-date. The most recently dated citation within the ATSDR Toxicological Profile on Mercury (ATSDR, 2024) is the same as it was in 1999, now 26 years out-of-date.
The most recent review of the toxicological literature relating to mercury vapor by a national or international environmental health agency was prepared by Health Canada (2006), which was subsequently published in the scientific literature by Richardson, et al. (2009).[24] If FDA had undertaken a thorough and effective review of all literature up to July 2009, as reported in their Final Rule, the Richardson, et al paper would have been identified. This is particularly true since the Richardson, et al paper is published in the journal Regulatory Toxicology and Pharmacology, a significant journal with high respect paid by the national and international regulatory community dealing with chemical exposures, such as mercury from dental amalgam.
It is also standard practice among practitioners of risk assessment to contact relevant national and international environmental health regulatory agencies to inquire of relevant unpublished reviews and documents. Had the FDA or their contractors followed that standard practice and contacted Health Canada to inquire about any relevant information, they would have been informed about both the document on mercury vapor and the subsequent journal publication. In fact, had the FDA or their contractors simply done an internet search on Health Canada’s web pages, they would have discovered Health Canada’s 1996 position paper on amalgam updating the reference exposure level for mercury vapor in the general population. Health Canada’s up-to-date REL (analogous to EPA’s RfC) of mercury vapor is 0.06 ug/m3, some five times lower than the out-of-date EPA RfC of 0.3 ug/m3, and more than three times lower than the ATDSR’s out-of-date MRL for mercury vapor of 0.2 ug/m3. Health Canada conducted another risk assessment in 2020 Health Canada conducted another risk assessment in 2020 confirming the 1996 recommendations.
In a review by Ratcliffe, et al. (1996), a series of criteria were developed to critically evaluate available epidemiological, occupational and toxicological studies of mercury, towards determining if post-1980s studies provided evidence to warrant revision of the REL for mercury. That review found several studies that were positive for sub-clinical impairment of the CNS. The study of Fawer et al. (1983), the primary basis of all existing REL values, did not meet the criteria on study quality established by Ratcliffe, et al.
Ratcliffe, et al. did not restrict their evaluation to studies of neurotoxicity. They also identified a variety of studies that were positive or suggestive of sub-clinical nephrotoxic effects, occurring in the same general dose range associated with sub-clinical CNS effects. Additional recent studies have also identified nephrotoxic, neurotoxic and immunotoxic effects associated with mercury exposure, reported at doses or exposure levels at or lower than the exposure levels associated with the Fawer study. As a result of the development of these factors, confidence in the current reference levels for mercury is low, at least outside of FDA.
This was recognized by the EPA, which in 2002, appended to their IRIS summary on elemental mercury (mercury vapor) the following statement:
Screening-Level Literature Review Findings – A screening-level review conducted by an EPA contractor of the more recent toxicology literature pertinent to the RfC for Mercury, elemental conducted in September 2002 identified one or more significant new studies. [Emphasis added]. And, that was 23 years ago. The research has continued to accumulate (See Appendix IV for a Table of relevant and recent literature (containing 158 unique references).
These more recent studies have most recently been reviewed and evaluated by Health Canada
(2006; see also Richardson et al., 2009).
b. The Fawer Study, Relied on by Both EPA and ATSDR, is a Study of Chloralkali Workers and not Appropriate for RfC or MRL Derivation
Most of the occupational studies underlying our knowledge of mercury vapor toxicity and, therefore, underlying all current RELs for mercury, were conducted on chloralkali workers. Although air-mercury concentrations are generally elevated among such workers, concomitant exposure to chlorine gas (Cl2) occurs. Data on airborne Cl2 levels in chloralkali plants were recently summarized by the European Union (EU, 2007). Cl2 levels in the air of chloralkali plants averages about 1 ppm (0.3 mg/m3) and ranges between 0 ppm and 6.5 ppm (0-19.5 mg/m3) depending on the specific work environment where sampling was conducted.
The concomitant exposure to Cl2 and Hgº effectively reduces worker exposure by decreasing the amount of airborne mercury available for inhalation and absorption. Mercury converts to HgC12 in the presence of Cl2 at room temperature (Menke and Wallis, 1980; Viola and Cassano, 1968). The inhalation absorption of HgC12 is only half or less of that of mercury (ATSDR, 1999; Viola and Cassano, 1968). Mercury deposition to the brain is also altered. Hg2+ (associated with HgC12) does not cross the blood-brain barrier as does Hgº (Lorscheider et al., 1995; Viola and Cassano, 1968). Following Hgº exposure, the red blood cell (RBC) to plasma Hgº concentration ratio typically ranges between 1:1 and 2:1 (WHO, 1991). However, much less Hgº is associated with RBCs in the blood of chloralkali workers (with Cl2 present).
Suzuki, et al. (1976), investigating Hgº-exposed chloralkali workers versus workers from two other industrial sectors (who were all exposed to mercury at similar airborne concentrations (0.01-0.03 mg/m3)), observed that the RBC to plasma Hgº concentration ratio in the chloralkali workers was only 0.02:1 whereas workers of the two other industries (with no concomitant exposure to Cl2), had RBC to plasma Hg concentration ratios between 1.5:1 and 2:1. A study by Viola and Cassano (1968) of rodents (rats, mice) exposed to Hgº alone or in the presence of Cl2, demonstrated reduced Hgº absorption in the presence of Cl2 and the deposition of Hgº to the brain of rodents exposed concomitantly to Hg0 and Cl2 was only 1/5th of that when exposure was to Hgº alone.
There is other evidence of the interaction of Cl2 with Hgº. Cl2 injection is employed as a direct mercury emissions control technology to reduce mercury levels in industrial stack emissions (Pavlish et al., 2003). Increasing chlorine quantity/concentration in the process improves the efficiency of mercury emission control (Richards, 2005). In the presence of chlorine, Hgº is converted to Hg2+, which precipitates with stack particulate matter that is subsequently removed (‘scrubbed’) from stack emissions.
It is evident, therefore, that all studies of uptake and toxicity of mercury exposure in chloralkali workers will be confounded by concomitant Cl2 exposure and, as a result, studies of chloralkali workers should not form the primary basis for a REL for mercury; the application and extrapolation of those results to other occupational groups and the general public, whose mercury exposure occurs in the absence of Cl2, is invalid. Even if they were valid, they did not consider or study women and children who weigh less and have greater vulnerabilities.
c. Current EPA Guidelines Require Updated Uncertainty Factors
The guidelines on risk assessment of neurotoxic agents (EPA 1998) clearly indicate that an uncertainty factor of ten should be applied when attempting to extrapolate a lowest-observed adverse-effect-level (LOAEL) to establish an REL, as is the case for studies of mercury vapor toxicity – the threshold cannot be determined from available studies. The guidelines on risk assessment of neurotoxic agents also clearly indicate that an uncertainty factor of ten should be applied to address inter-individual variability in susceptibility to the toxic effects of neurotoxins such as mercury vapor. This would create a total uncertainty factor adjustment of 100. The EPA RfC for mercury vapor, which predates EPA’s 1998 guidance on the risk assessment of neurotoxins, only applied a total uncertainty adjustment of thirty, an adjustment now out of compliance with EPA policies.
Further modifying factors may also be considered by the EPA when they re-assess mercury vapor neurotoxicity, that modifying factor addressing other deficiencies and limitations in the toxicological database on mercury vapor. Those deficiencies and limitations may include, but not be limited to, the following:
i. Sex Differences in Mercury Pharmacokinetics
Recent evidence indicates clear sex differences in uptake, distribution, and excretion of mercury. Studies indicate that males metabolize and eliminate mercury more quickly than do females and that, after exposure, mercury tends to be distributed differently in males and females, with a greater proportion of mercury targeting the CNS (i.e., brain) of females and to the kidney in males. Further, it appears to be retained for a longer time in females and thus be potentially more toxic in females.
Several authors have indicated that sex is an important factor in the metabolic and toxicologic response to exposure to chemicals (Calabrese, 1986; Silvaggio and Mattison, 1994; Gochfeld, 1997; Iyaniwura, 2004). There is evidence that males and females respond differently to mercury exposure, in terms of uptake, distribution, and toxicity. As discussed below, studies examining both sexes have exhibited differing accumulation patterns in males and females, and faster elimination rates in males. These differences may result in variable, sex-related toxic response to mercury exposure. The available data, however, are limited and inadequate to reliably quantify sex-related differences in toxicity.
It should be noted that both organic (methyl Hg) and inorganic forms of mercury were considered in this review of sex-specific response because once across the blood-brain barrier the ultimate biochemical fate of the ionic mercury moiety (Hg2+ from organic and inorganic Hg) is identical (Lorscheider et al., 1995). FDA completely fails to account for this additional body burden in women when comparing exposure to the RfC and MRL.
Hongo et al. (1994) examined urinary Hg excretion by university staff and students who were occasionally exposed to mercury vapor over a period of six years. Regression analysis indicated that the mercury vapor exposure level was the major variable predicting urinary mercury excretion, but sex (along with age and the presence of amalgam fillings) were also reported to be important factors. They did not, however, specifically quantify the sex-related differences.
Jokstad (1990) surveyed the Norwegian Dental Association to assess the significance of potential sources of mercury exposure. Urinary mercury excretion values were correlated to answers on the survey. In addition to correlations between environment and practice characteristics and mercury excretion values, the data indicated that urinary Hg excretion might be sex-dependent, due to the fact that the mean urinary mercury levels of 849 participants were slightly lower in women compared to men (40 nmol/L versus 44 nmol/L). When a group of female assistants with higher exposures were excluded from the analysis, the average urinary mercury concentration for women dropped to 38 nmol/L. The authors reported, “[n]either the length of work experience, nor the years in the current office facility correlate[d] with the urinary Hg levels.” While there was a correlation between urinary mercury concentrations and the number of hours spent per week in the clinic for the entire group and for the male participants, this correlation was not observed when female participants were evaluated alone. The mean mercury concentrations for females remained relatively constant and, for the most part, were lower than those measured in the male participants, especially at the higher exposure levels. The authors did not offer a definitive conclusion as to whether their results support sex-dependency in absorption or excretion.
At an annual American Dental Association (ADA) meeting, Kaste, et al. (1992) presented a study of dentists and dental assistants who had been evaluated for Hg exposure. Over 4000 participants (7.6% women) answered questionnaires and provided urine samples. There was a small difference in average urinary mercury concentration (4.9 µg/L in women and 6.3 µg/L in men). This variation might, however, be attributable to the number of years of exposure as it was reported that females had an average of 8.2 years in practice while males averages 19.2 years.
Pamphlett, et al. (1997) compared the uptake of inorganic mercury by motor neurons in male and female mice and measured mercury concentrations in their kidneys. Significantly more neurons contained mercury granules in female mice than in male mice, and kidneys of male mice had significantly higher amounts when compared to females. The authors concluded that the decreased deposition of mercury in the kidneys of the female mice resulted in an increase in circulating mercury, which was available for neuron uptake.
Pamphlett & Coote (1998) were interested in identifying the lowest dose of mercury vapor that resulted in mercury deposition in neurons, and in determining if the neurons of females were more susceptible to mercury vapor toxicity than neurons of males. After a 50 µg/m3 dose, mercury was observed in the spinal motor neurons of female mice at half the exposure time (6 hours) necessary for it to be observed in the spinal motor neurons of male mice (12 hours).
Nielsen & Anderson (1990) investigated the effects of different dose levels and routes of administration on whole body retention and relative organ distribution of mercury chloride in two strains of female mice. In addition, the authors investigated sex differences in the distribution of mercury chloride by comparing their results to a previous study with male mice (Nielsen & Andersen, 1989). This comparison showed that similar fractions of mercury body burden were distributed in the liver of males and females, while a significantly larger fraction of Hg body burden was deposited in the kidneys of the male mice than in female mice.
Thomas, et al. (1986) examined the integrated exposures of tissues of female and male rats to organic and inorganic mercury. While whole body comparisons indicated that integrated exposures of males and females to inorganic mercury were equal, this study demonstrated that the integrated exposure of the brain of female rats to inorganic mercury was 2.19 times that of the males. This finding suggested that there was a sex-related difference in the accumulation and/or retention of inorganic mercury in the central nervous system.
Miettnen (1973 as cited in Thomas, et al. 1986) reported that, in humans, the whole body half time for mercury elimination following ingestion of protein bound mercury chloride was faster in females than in males.
Hirayama & Yasutake (1986) and Yasutake & Hirayama (1988) studied mice to evaluate the mechanisms for sex-related differences in the in vivo fate of methyl mercury. A single administration of methyl mercury chloride in mature mice resulted in higher levels of urinary mercury in males than of females. Five minutes after exposure, mercury levels in male kidneys were higher than in female kidneys and these higher male concentrations were still in evidence after 24 hours. Lower mercury values were reported in other tissues of males when compared with females. After 24 hours, the mercury levels in urine were 6.5 times higher in males than in females. The levels of mercury in kidneys for males were higher than in females whereas the females had higher mercury levels in the brain, liver and plasma. Castrated males had mercury tissue levels similar to females except in the brain and castrated females exhibited decreased urinary excretion of mercury. The authors concluded, “tissue distribution and urinary excretion of the administered methyl mercury seem to be subject to sex hormone control. This study demonstrates that the metabolism and elimination of methyl mercury occurs significantly faster in males and that the sequence of events leading to urinary excretion of methyl mercury may proceed under the control of sex hormones.”
Magos et al. (1981) compared the sensitivity of female and male rats to methyl mercury. “After identical doses the brains of females always contained more mercury than those of males. Female rats developed more intensive co-ordination disorders and after five doses they had more extensive damage in the granular layer of the cerebellum than males.” However, the regional distribution of mercury within the brain was the same in males and females. The elimination rate in male kidneys was found to be significantly faster (16-day half-life) than the elimination rate for female kidneys (37 day half-life).
Nielsen and Andersen (1991) found the route of methyl mercury administration did not affect the whole-body retention of mercury significantly but that female mice retained more mercury than did male mice. Kidney deposition in males was twice that in females, and the male mice excreted mercury significantly faster than did the females.
ii. Genetic predisposition to Hg toxicity
A variety of studies in animals (Aten, et al., 1992; Druet, et al., 1978; Hirszel, et al.,
1985; Hultman and Enestrom, 1992; Matsuo, et al., 1987; Michaelson, et al., 1985; Pelletier, et al., 1990; Pusey, et al., 1990; Roman-Franco, et al., 1978; van der Meide, et al., 1993) (see reviews by Silbergeld, et al., 2005; Nielson & Hultman, 2002; ATSDR, 1999) demonstrate the occurrence of autoimmune glomerulonephritis upon exposure to mercury in genetically susceptible animals.
Autoimmune glomerulonephritis results in observed proteinuria due to autoantibodies reacting with renal tissues. Some human evidence supports the existence of an immunologically mediated renal impact of mercury, with deposition of IgG, immune complexes and/or complement C3 along the glomerular basement membrane (Lindqvist, et al., 1974; Tubbs, et al., 1982). This has been interpreted as evidence of a potential genetic predisposition to immunologically mediated renal response to mercury exposure, although the existence of a genetic polymorphism coding for the requisite genetic susceptibility has not been reported.
Echeverria, et al., (Echeverria, et al., 2006, 2005; Woods, et al., 2005; Heyer, et al., 2004) have recently identified polymorphisms in genes encoding for brain-derived neurotrophic factor (BDNF). Various detriments in neurobehavioral performance (Echeverria, et al., 2006, 2005) and in symptoms and mood (Heyer, et al., 2004) were associated with the presence of the BDNF polymorphism (frequency = _25-35% among study subjects (193 male dentists; 233 female dental assistants)), independent of mercury exposure level. The combined effects of the polymorphism and mercury exposure appeared to be additive. These results suggest that the presence of the polymorphism does not necessarily put persons at risk of an enhanced toxic response to mercury exposure. Rather, persons with the polymorphisms might respond to mercury exposures similarly to those without, but from a diminished starting point with respect to neurobehavioral performance.
The presence of a polymorphism for coproporphyrinogen oxidase (CPOX4; frequency=15% of subjects in Woods, et al. (2005); and 25% of study subjects in Echeverria, et al. (2006)) has also been observed and is associated with detriments in neurobehavioral response independent of mercury exposure. As with BDNF, the influence of the CPOX4 polymorphism and mercury exposure appeared to be additive.
iii. Fetal Effects of Mercury
Although multiple studies have identified dose-dependent increases in fetal brain mercury concentrations, dose-response data related to fetal neurotoxicity are non-existent, with the exception of a single study (Morgan, et al., 2002) that reported a no-effect-level of 108.5 ng Hg/fetus (whole body) in rats. As a result, the potential for fetal exposure and effects must be considered in REL development, but at present must be addressed as a limitation of the database available for the determination of a REL for mercury.
The uptake and distribution of mercury in the fetus following maternal exposure has been extensively reviewed (ATSDR, 1999; WHO, 2003). Animal studies suggest that the CNS is sensitive to prenatal mercury exposure. However, clear dose-response data in relation to maternal inhalation exposure to mercury is lacking. In addition, available data relate to mercury air concentrations two to three orders of magnitude greater than that generally encountered in the non-occupational environment. High quality epidemiological data (e.g., with good exposure data and control of confounding factors) is lacking concerning the potential for CNS effects in children exposed in utero. Therefore, while there is evidence to demonstrate that fetal exposure does occur, and to suggest potential concern for fetal neurobehavioral effects following maternal inhalation exposure to mercury, data are lacking to quantify potential risks.
As mercury can readily cross the placenta (WHO, 2003), fetal exposure represents a concern in association with the inhalation of mercury by pregnant women (WHO, 1991; Drasch, et al., 1994; Yang, et al., 1997; Vimy, et al., 1990; Yoshida, et al., 1986, 1990). No hepatic or renal effects have been noted as a result of in utero exposure despite the fact that the liver and kidney of the fetus accumulate the highest levels of mercury (Drasch, et al., 1994; Morgan, et al., 2002; Yoshida, 2002; Yoshida, et al., 2002). Many recent studies have examined the effects of in utero mercury exposure and have pointed to potentially irreversible neurological effects as the key concern (Ramirez. et al., 2003). This highlights the sensitivity of the developing CNS to mercury, with one author attributing this sensitivity to its slow elimination from these tissues (Yoshida et al.,1999).
There have been a few studies published since the previously cited reviews were completed. Yoshida, et al. (2005) repeatedly exposed pregnant mice of metallothionein (MT) null and wildtype strains to mercury at concentrations of 0.5 mg/m3 and 0.56 mg/m3, respectively, for 6 h/day from gestational day (GD) 1 through 18. Mercury concentrations in the brain and kidney in the offspring were found to be significantly higher in the exposed groups (MT-null and wildtype) than in the controls. In the brain, mercury concentrations in the exposed males were not significantly different between the two strains, but the exposed MT-null females had significantly higher levels of mercury than the wildtype females. A histological examination did not reveal any abnormalities in the nerve tissues of the exposed mice regardless of strain or sex of the offspring.
Mercury-exposed MT-null male mice exhibited a significant decrease in total locomotor activity; a learning disability in the passive avoidance response in females; and a retarded acquisition in the Morris water maze in females, as compared with the controls. The authors concluded that MT may play a protective role for neurological effects associated with in utero mercury exposure, with its influence being more pronounced in females.
Another recent study examined the disposition and toxicity of inhaled mercury in rats and the potential adverse effects on reproductive outcomes (Morgan, et al., 2002). Rats were exposed to 0, 1, 2, 4 or 8 mg Hg/m3 for 2 h/day from GD 6 through 15. Maternal toxicity was noted in the 4 and 8 mg Hg/m3 groups, which was characterized as a concentration-related decrease in body weight gain and mild nephrotoxicity. The accumulation of mercury in fetuses was found to be dose dependent, however, no statistically significant effects on fetal brain weights or on fetal body weights were noted even with fetal mercury concentrations reached a mean of 108.8 ng Hg/fetus (whole body) on GD 10 (the only day on which whole body burden was examined) and 1.93 ng/brain by GD 15. The authors also noted a dose-related increase in levels of mercury in the fetal brain. While no effects were noted in the offspring following in utero exposure, a significant increase in the number of resorptions was noted in the highest dose group, where maternal toxicity was observed. In the same dose group, post-natal litter size and body weights of neonates were significantly less than controls. The direct maternal toxicity reported at this exposure level confounds the interpretation of effects on reproductive outcomes.
A study in humans examined the presence and levels of total mercury in cord blood and meconium as an indicator of prenatal exposure and the potential for neurodevelopmental effects (examined using cognitive adaptive tests and clinical linguistic auditory milestone scale CATS/CLAMS) (Ramirez, et al., 2003). The authors did not provide details concerning the source of the exposures to mercury (both elemental and methyl mercury) in the study but noted that there was likely some exposure to methyl mercury via the diet due to the consumption of fish. The study reported that mercury levels in hair and cord blood were negatively correlated with CATS/CLAMS results in both the control and exposed groups at two years of age. However, those exposed also had documented indicators of mercury presence at birth (e.g., presence of mercury in the meconium) and, therefore, the authors suggested that prenatal exposure, and not necessarily current exposure was the cause of the observed neurodevelopmental effects in children from birth to two years of age. While this study suggests that in utero exposure may result in neurological effects, these results should be interpreted with caution, as the authors did not control for confounding variables, such as concomitant exposure to other neurotoxicants and nutritional deficiencies.
10. Mercury Has Been Identified in a Large Number of Peer Reviewed Studies As Being a Likely Cause of the More Prevalent Neurological Disorders including Alzheimer’s Disease (AD), Severe Autism, Multiple Sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), and Parkinson’s Disease (PD). It also causes Kidney Dysfunction, Hearing Loss, Allergy, and Periodontal Disease.
As a preliminary matter, we notice that FDA declined to consider review articles on the ostensible basis that they present no new empirical data for consideration. FDA then relies on assurances of amalgam safety announced in a 2004 review article prepared by LSRO as the ostensible basis for generally refusing to consider articles published prior to LSRO’s review. It seems as a matter of simple objectivity that review articles are either to be considered or they are not. If FDA is willing to consider LSRO’s review article, it should consider the dissenting opinions set forth in some of the review articles identified herein. It appears to us that an objective FDA would heed the rejection of the FDA’s White Paper by FDA’s own hand-picked Joint Panels in 2006 and question the proclamations of safety previously announced by LSRO in 2004. Instead, FDA rejects the announcements of its advisory panels and accepts without question the questionable views of LSRO. Following is a more robust discussion of the literature associating various diseases and conditions with exposure to mercury.
a. Alzheimer’s Disease (AD)
There are multiple neurological disorders for which the cause remains unknown. The clinical pictures of several of these are most interesting when considered in light of the documented neurotoxicity of mercury and the potential for neurotoxicity from mercury/silver fillings.
Despite the protests of the FDA and the ADA, the science confirms that these fillings emit significant levels of neurotoxic mercury, and mercury is injurious to human health. This mercury from fillings would certainly exacerbate and contribute to the cause of AD, MS, PD, autism and ALS. The synergistic effects of mercury with many of the toxicants commonly found in our environment make the danger of mercury unpredictable and possibly quite severe, especially any mixture containing elemental mercury, organic mercury, and other heavy metals such as lead and aluminum.[25]
The literature linking mercury to AD has accumulated over the last four decades. In 1986, Ehmann reported that samples of AD brain analyzed by neutron activation had significantly elevated amounts of mercury in every area analyzed. In some areas such as the cerebellar hemisphere mercury levels were ten-fold greater in AD than controls (table 4).[26] The elevated mercury imbalance in AD brain was confirmed in a follow up studies by Thompson and others (1998).[27] Through cell fractionation, Wenstrup was able to trace the accumulation of mercury into the mitochondria, the powerhouse of the cell, producing essential proteins (1990).[28] These papers were all published in high quality scientific journals with expertise in reviewing such analytical data.
Later a paper was published in the Journal of the American Dental Association (JADA) that supposedly refuted these findings (Saxe et al, 1995).[29] It should be noted that JADA is a journal with no expertise in reviewing analytical chemistry or neurology and has been highly criticized for its unwarranted conclusions. However, even in this paper, the mercury levels in the brains of Catholic nuns showed many of the Sisters had levels of mercury that should be considered toxic by any scientific standard. Mercury is neurotoxic and is known to be the most potent causation of oxidative stress, a biochemical state that is widely known to exist in AD and other neurological illnesses. The Saxe et al study is reviewed in more depth below.
When exposed to normal brain tissue homogenates or neurons in culture Hg2+ (a/k/a, mercury (II) or mercuric mercury) can produce many of the same biochemical aberrancies found in AD brain. Rats exposed to mercury vapor show some of these same abnormalities in their brain tissue. Specifically, the rapid inactivation of the brain thiol-sensitive enzymes (tubulin, creatine kinase and glutamine synthetase) occurs after: (a) the addition of low micromolar levels of Hg2+, (b) exposure to Hgº or, (c) the addition of Thimerosal (ethylmercurythiosalicylate sodium salt). Moreover, these same enzymes are significantly inhibited in the AD brain. Exposure of neurons in culture to nanomolar levels of Hg2+ has been shown to produce three of the widely accepted pathological diagnostic hallmarks of AD. These AD hallmarks are elevated amyloid protein, hyper-phosphorylation of Tau, and formation of neurofibrillary tangles (NFTs).[30]
In 2001, at University of Calgary Leong, et al, published a paper that included a video clip showing the disruption of tubulin-neurofibril interaction that represents how mercury, and only mercury, as opposed to other metals, can cause synaptic neurodegeneration by destroying neuron growth cones.[31] The cultured neurons exposed to low levels of mercury degenerated in a manner indicative of lesions observed in AD brain. This video clip can be viewed on YouTube. It is important to note that the level of mercury added to the cell culture in this video was one hundred times lower than is typically detected in the cerebral spinal fluid of those with mercury/silver amalgam tooth fillings. The Leong paper is important as it demonstrates that mercury, and only mercury, produces neurofibillary tangles (NFTs) the major diagnostic hallmark of AD. This paper was omitted from FDA’s consideration because it is an in vitro study, but it is an important paper because it confirms the hypotheses of other papers. The work of Leong et al supports the earlier reported mercury specific destruction of the viability of brain tubulin.[32] Professor Boyd Haley concluded in 2003 that “mercury and other blood-brain permeable toxicants that have enhanced specificity for thiolsensitive enzymes are the etiological source of AD. Included in this category are other heavy metals such as lead and cadmium that act synergistically to enhance the toxicity of mercury and organic-mercury compounds.”[33] The demonstrated toxic synergy of mercury with other heavy metals is a concept completely omitted from consideration in FDA’s Final Rule.
Haley found that mercury is the only heavy metal and apparently the only toxin of any kind that can cause many of the biochemical abnormalities found in the AD brain. The demonstrated synergistic potentiating of mercury toxicity by other heavy metals (lead, cadmium, silver, etc.) explains why a direct correlation between mercury levels alone and severity of AD-like brain damage is difficult to demonstrate.
Studies done on about five hundred sets of identical twins from WWII veterans show that AD is definitely not a directly inherited disease, as it requires a toxic insult.[34] Certainly, all the information and scientific studies point to toxin(s) as the major cause of AD. Ely confirmed substantial release of mercury from in situ amalgams and estimated the AD population would grow from its 2001 level of 4 million individuals to 14 million based upon population age alone.[35] This enormous increase will devastate any health care system, as the cost of providing for even the 4 million AD patients at present dwarfs the total cost of dental care.
Mutter detailed why the apolipoprotein-4 genotype represents a genetic susceptibility to mercury toxicity as a pathogenetic factor and a moderator of AD.[36] Mutter also demonstrates that persons of African descent have a much higher level of the susceptible APOE4 gene. This may explain why AD is more prevalent in those with an African heritage.
In 1997, APO-E4 was identified as a significant risk factor for early onset of AD with APO-E2 genotype being identified as protective against AD.[37] Several subsequent papers did not clarify the reason. APO-E has 299 amino acids with different ratios of cysteine and arginine at position 112 and 158. APO-E2 has 2 cysteines, apo-E3 one cysteine and one arginine, and APO E4 two arginines.[38] An arginine, unlike cysteine, lacks the sulphydryl (SH) groups to potentially bind bivalent metals such as mercury, lead, copper or zinc, it would be logical to suspect the possibility of increased metal accumulation in those chronically exposed individuals without the APO-E2 genotype. Godfrey et al 2003 found there was a statistically significant increase in adverse effects in those patients having APO-E4/4 and APO-E 3/4 when they were chronically exposed to mercury.[39] Godfrey went on to explain why this occurs:
According to Saunders, the underlying reason for the apo-E-associated differences in AD susceptibility remains a mystery. However, a logical biochemical explanation has been proposed by Pendergrass and Haley, based on the different amino-acid configurations of the three apo-E isomers and their potential relevance to mercury elimination. Only ɛ2 (with two cysteine -SH groups), and to a lesser extent ɛ3 (with one-SH group), are able to bind and remove mercury from the brain and cerebrospinal fluid. This would oppose accumulation of mercury.[40]
Godfrey added: Another aspect of AD pathology is the evidence that enhanced mitochondrial damage occurs in AD and ɛ4 genotype. Mercury is very destructive at the mitochondrial level where catalase can demethylate organic mercury species into highly reactive inorganic mercury. Inorganic mercury is also an extremely potent enzyme inactivator. Furthermore, chronic micro-mercurial toxicity specifically from dental amalgam has been documented and successfully treated by removal of amalgam and medical detoxification in 796 patients.
Still, not all research results agree with mercury’s causal role in AD. Elevated mercury was not found in seven different regions of AD brains compared to controls. However, the “controls” had possessed three amalgam surfaces whereas the AD subjects had six, likely obscuring any differences. Saxe et al. reporting on the mental health of 129 nuns, found no difference between those with amalgam and controls. However, 72% of the controls had no posterior teeth, and the remainder had a mean of only three teeth. All 129 could, therefore, have had a similar previous amalgam history and the half-life of mercury in the brain is measured in decades. This paper’s conclusions, published in a dental trade journal, are at variance with those of another paper in the same journal on risk factors affecting dentists’ health. The authors identified 3 factors with equally high statistical values (i.e. p < 0.001), namely, a mercury spill in the dental office, manual amalgamation, and the dentists’ own amalgam status.[41]
Wojcik’s research (2006) supported a correlation between a genetic inability to eliminate mercury when the APO-E4 allele has been inherited and an increased incidence of common symptoms and signs of chronic mercury toxicity. [42] Thus, the increased likelihood of AD in APOE4 is almost certain to be caused by mercury exposure, a known and powerful neurotoxin. As shown by Khatoon et al 1989,[43] Wojcik 2006 stated:
Two very important brain nucleotide binding proteins, tubulin and creatine kinase (CK), showed greatly diminished activity and nucleotide binding ability in the AD brain tissues versus age-matched control brain samples.22 Both tubulin and CK are proteins that bind the nucleotides GTP (guanosine-5′-triphosphate) and ATP (adenosine-5′-triphosphate), respectively. After testing numerous heavy metals, it was observed that, in the presence of EDTA, or other natural organic acid chelators, only Hg2+ mimicked the biochemical abnormalities observed for tubulin in the AD brain homogenates examined. This was first done by adding low amounts of Hg2+ and other toxic heavy metals to homogenates of normal brain tissue in the presence of various metal chelators.
There are a plethora of additional scientific articles linking mercury to Alzheimer’s Disease[44] See Appendix I for additional and newer evidence.
With the weight of the evidence there can be little doubt that mercury, more likely than not, plays a large role in AD and certainly would exacerbate it. Certainly, FDA’s Final Rule completely fails to address, much less refute, the concerns raised by this existing research.
NIH refuses to fund studies that may compromise its–and FDA’s–long-held (but scientifically unsupported and unsupportable) claims touting the safety of amalgams. Specifically, NIH has improvidently refused to consider mercury exposure as the cause of AD. This is done, in the opinion of many, to protect industrial interests in developing a drug to treat elevated beta-amyloid conditions. Perhaps, in the near future, with help from international researchers, AD will be renamed, “mercury -induced dementia.”
b. Parkinson’s Disease (PD)
Scientific studies have suggested associations between mercury and neurological disease. These studies justify avoiding unnecessary mercury exposure. For example, one epidemiologic study correlates systemic mercury levels with increased risk of idiopathic PD.[45] John Pearlman, M.D., reported that a 50-year-old female patient had mercury/silver fillings removed and suddenly developed permanent neurological impairment that was ultimately diagnosed as PD. She was confined to a wheelchair.45 Manufacturers of mercury/silver fillings warn that removal can be dangerous.
c. Multiple Sclerosis (MS)
MS was first commonly identified in the 19th century during the time in which mercury/silver fillings came into common use. Unpublished anecdotal evidence indicates that a significant number of, but certainly not all, MS victims who have their mercury/silver fillings removed resolve (spontaneous remission) or improve gradually. By 1993, forty-two MS victims had filed adverse reaction reports with the FDA. Four of these were cured and twenty-nine improved. There is toxicological evidence that mercury poisoning victims (from sources other than fillings) and MS victims share similar symptoms. The Encyclopedia of Occupational Health and Safety discusses the symptoms of chronic mercury poisoning, in part, as follows:
Nervous system involvement may occur with or without gastrointestinal symptoms, and may evolve in line with two main clinical pictures: (a) fine intention tremor reminiscent of that encountered m persons suffering from MC.
The most frequently encountered symptoms resemble those presented by persons with MS except there are no nystagmus and the two conditions have a different serology and different clinical courses.
In 1966 Baasch concluded, based on sometimes severe neuro-allergic reactions in acrodynia (pink disease) and his own observations of neurologic patients, that MS was an adult form of acrodynia (pink disease) and a neuro-allergic reaction, in most cases, caused by mercury from amalgam fillings.[46] Baasch demonstrated in great detail that facts concerning the geographical and age distribution, pathological development, and symptomatology of MS were all consistent with amalgams being the primary cause of the disease. He reported several specific cases and cited ongoing studies that showed cessation of progression and improvement of resolution of MS after removal of amalgam fillings.
In a very detailed study, Craelius in 1978 showed a strong correlation (P<0.001) between MS death rates and dental caries.[47] The data demonstrated the improbability that this correlation was due to chance. Numerous dietary factors were ruled out as contributing causes.
A hypothesis presented in 1983 by T.H. Ingalls, M.D. proposed that slow, retrograde seepage of mercury from root canals or amalgam fillings may lead to MS in middle age.[48] He proposed a correlation of unilateral MS symptomatology with ipsilateral amalgam-filled teeth. He also re-examined the extensive epidemiological data that show a linear correlation between death rates from MS and numbers of decayed, missing, and filled teeth. Ingalls suggested that investigators studying the causes of MS should carefully examine the patients’ dental histories.[49] Furthermore, Dr. Ingalls’ hypothesis included other environmental exposures to mercury. In 1986, he published data supporting his hypothesis that clearly demonstrate endemic clustering of MS in time and space over a 50-year time span that could be directly correlated to exposure to mercury.[50] Another study (Ahlrot-Westerlund 1987) found that MS patients had 8 times the normal level of mercury in their cerebral spinal fluid as compared to neurologically healthy controls.[51]
In a 1990 study, the University of Aarhus, Denmark, Department of Neurobiology, conducted an experiment in which three vervet monkeys received occlusal amalgam fillings, three others maxillary bone implants of amalgam, and three untreated monkeys served as controls, in order to trace possible accumulations of mercury. One year later, tissue sections from different organs were subjected to silver amplification by auto-metallography and analyzed at light and electron microscope levels. It was found that amalgam fillings (total 0.7-1.2g) cause deposition of mercury in the following tissues: spinal ganglia, anterior pituitary, adrenal, medulla, liver, kidneys, lungs, and intestinal lymph glands. In the monkeys with maxillary silver amalgam implants (total .1-.3g), mercury was found in the same organs with the exception of the liver, lungs, and intestinal lymph glands. Organs from the three control animals were devoid of precipitate. These results strongly support what has been suggested previously–that dental fillings in primates cause absorption of mercury released from amalgam fillings through the lungs and the intestinal tract, and that mercury is distributed to most organs and will eventually be found in the central nervous system. The study also shows that silver released from the corroding filling is not absorbed.[52]
In a 1998 study, Dr. Svare and associates analyzed, the expired air of a group of 48 persons for its mercury content, 40 with, and eight without dental amalgam restorations, before and after chewing55. Expired air samples were collected in polyethylene bags, and a known quantity of each was pumped into the mercury detector for measurement. The results showed that subjects with dental amalgams had higher pre-chewing mercury levels in their expired air than those without amalgams. After chewing, these levels were increased an average of 15.6-fold in the former and remained unchanged in the latter group. It was therefore concluded that in situ dental amalgams can indeed increase the level of mercury in expired air.
A paper written in 1994 by Dr. Siblerud of the Rocky Mountain Research Institute, Inc., investigated the hypothesis that mercury from silver dental fillings (amalgam) may be related to MS.[53] It compared blood findings between MS subjects who had their amalgams removed to MS subjects with amalgams. MS subjects with amalgams were found to have significantly lower levels of red blood cells, hemoglobin and hematocrit compared to MS subjects with amalgam removal. Thyroxine levels were also significantly lower in the MS amalgam group and they had significantly lower levels of total T Lymphocytes and T-8 (CDS) suppressor cells. The MS amalgam group had significantly higher blood urea nitrogen and lower serum IgG. Hair mercury was significantly higher in the MS subjects compared to the non-MS control group. A health questionnaire found that MS subjects with amalgams had significantly more (33.7%) exacerbations during the past twelve months compared to the MS volunteers with amalgam removal.
An article developed by the MELISA Foundation in March of 2005, noted that MS is caused by the erosion of myelin, a substance which helps the brain send messages to the body. Metal particles entering the body can bind to this myelin. For those who are hypersensitive, this myelin-metal bond comes under attack from the immune system. In such cases, the progression of MS can be halted by removing the source of the metal. The role of myelin is one of the few facts on which those who study MS maintain agreement. The MELISA Foundation has developed what they believe is a breakthrough in understanding in MS: the link between metal allergy and the erosion of myelin.[54] They believe that they have also been able to prove that the myelin erosion can be halted if the source of the allergy is removed. Hypersensitive reactions are triggered by metal particles entering the body of a person allergic to the metal in question. These particles then bind to the myelin, slightly changing its protein structure. In hypersensitive people, the new structure (myelin plus metal particle) is falsely identified as a foreign invader and is attacked; an autoimmune response. Arrows point to the “myelin plaques” in the brain, common in patients with MS. Such plaques can be the result of metal allergy. The MELISA Foundation has seen patients with MS make a partial, and, in some cases, a full recovery by removing the source of metal – often dental fillings.[55]
Mercury has been documented to accumulate in the very areas of the nervous system from which most dramatic clinical symptoms of MS originate. Specifically, motor neurons accumulate more mercury than sensory neurons, and motor symptoms are seen to predominate over sensory symptoms in MS. Although more research needs to be done in this area, these results suggest dental mercury exposure from amalgams, as well as from any other chronic low-grade mercury exposure, must be given very serious consideration as possibly playing a role in the etiology of MS in such patients and more likely is the major cause of most MS. Genetic variability and individual ability to excrete mercury probably plays a role.[56]
In conclusion, the causation of MS is probably multi-factorial. Mercury is certainly one cause and probably the major cause of this disease.
d. Amyotrophic Lateral Sclerosis (ALS)
ALS, more commonly known as Lou Gehrig’s disease, is another “idiopathic” neurological disorder. ALS was first identified a few years after mercury/silver fillings came into common use. The clinical picture is quite interesting when considered in light of the documented neurotoxicity of mercury and the potential for neurotoxicity from mercury/silver fillings, often referred to as amalgam. Like MS, some people with ALS have found that their condition improved dramatically upon the removal of their amalgam fillings. Others have not improved which may be the result of poor technique resulting in high exposure to mercury during the removal process or they may be genetically a nonexcreter of mercury.[57] The correlation to mercury exposure was first suggested by Brown in 1954..[58]
A 1961 study of eleven cases of chronic mercurialism from consumption of bread treated with a mercury-containing fungicide presented neurological symptoms akin to ALS with some more closely resembling progressive muscular atrophy. The paper concluded:
1. The same causative factor was operative in all these cases, suggesting that ALS and progressive muscular atrophy are nosologically identical.
2. ALS should not be considered a disease entity but rather a syndrome of variable etiology.
3. Chronic mercurialism is a possible etiologic factor in ALS.” (emphasis added)”[59]
A 1978 report by Barber is also noteworthy. This involved two employees in a mercury oxide manufacturing plant who developed previously non-existent neurological symptoms resembling that of ALS.[60] An additional nineteen employees precipitously developed signs and symptoms which may be regarded as the early onset of a symptom complex of mercury intoxication that would likely have progressed to the ALS-like syndrome if the progression had not been interrupted by removal of the individuals from exposure to mercury. All symptoms, signs, and laboratory findings returned completely to normal after approximately three months in a mercury free work environment.
In 1983 the Journal of the American Medical Association reported of a 54-year-old man with symptoms resembling ALS after a brief but intense exposure to elemental mercury which resolved shortly thereafter, as his urinary mercury levels fell.[61] This man who had breathed mercury vapor while “salvaging the liquid mercury from industrial-grade thermometers” developed symptoms so similar to that of ALS that his neurologists gave him a “presumptive diagnosis of ALS.” The man’s physicians confirmed his exposure to mercury with a urine test “several weeks” after his exposure, which registered 99 micrograms of mercury per liter of urine, an alarmingly high concentration. Two months later, the man had recovered nearly completely. His “neurological findings were completely normal.” His urine test indicated his mercury level had dropped to 29 micrograms, which is still much higher than the norm of 4 to 5 micrograms per liter. And “several weeks” later his mercury level had fallen to 8 micrograms.
A 1989 a Japanese study was done on ALS victims in the vicinity of the biggest mercury mine in Japan. That study found mercury at higher levels in ALS victims than in controls. They followed this with a study in 1990 which compared the mercury and selenium content in the hair of thirteen (13) ALS cases using neutron activated analysis and concluded that mercury with a low content of selenium might be one of the environmental factors.[62]
There are other studies indicating a connection between mercury and ALS – a case report describing recoveries from ALS after the removal of mercury/silver fillings,[63] and another case report of ALS developing after the accidental injection of mercury.[64] A 1990 study in the U.S. also involved neutron activated analysis of the brain, spinal cord, blood cells, serum, and nails of ALS victims compared to controls. Imbalances were detected in a number of trace and minor abundance elements in the tissue of ALS patients and more widespread changes were noted in the concentrations of mercury. The authors cautioned that the variation in mercury concentrations need not necessarily indicate active toxicity, as it could merely represent an enlarged pool of detoxified mercury or perhaps a labeling of a specific cellular ligand by mercury in ALS.[65]
Unlike MS there are not many adverse reaction reports to the FDA involving ALS and the removal of mercury silver fillings and it is very important to note there are individuals who have ALS and have never had mercury/silver fillings. So while mercury may be one cause of ALS as the foregoing suggests, it certainly is not the only one.
Despite this considerable evidence linking ALS and mercury, the NIH has refused to fund further research into mercury as a possible cause of this tragic disease which disables and- usually within two to five years– kills five thousand people each year.
e. Severe Autism
A 2009 epidemiological study strongly associates prenatal mercury exposure from maternal dental amalgams with significantly increased rates of severe autism.[66] Proclaiming human fetal safety based on minimal animal data, FDA inexplicably fails to explain how this important study eluded FDA’s attention.
Holmes, et al (2003), found that mothers in the autistic group had significantly higher levels of mercury exposure through Rho D immunoglobulin injections and amalgam fillings than control mothers. Within the autistic group, hair mercury levels varied significantly across mildly, moderately, and severely autistic children, with mean group levels of 0.79, 0.46, and 0.21 ppm, respectively. Hair mercury levels among controls were significantly correlated with the number of the mothers’ amalgam fillings and their fish consumption as well as exposure to mercury through childhood vaccines, correlations that were absent in the autistic group. Hair excretion patterns among autistic infants were significantly reduced relative to control. These data cast doubt on the efficacy of traditional hair analysis as a measure of total mercury exposure in a subset of the population. In light of the biological plausibility of mercury’s role in neurodevelopmental disorders, this study provides further insight into one possible mechanism by which early mercury exposures could increase the risk of autism. [See also, Mutter J, Mercury and autism: Response to the letter of K. E. v. Muhlendahl, Int. J. Hyg. Environ. Health 208 (2005) (“Effective excretion of mercury will lead to higher hair, blood and urine mercury levels in a population that is being exposed to mercury at a constant, chronic, low level. The problem comes when those who do not effectively excrete mercury, become exposed to a large dose, such as infants already exposed to mercury during pregnancy and who in addition received thimerosal containing hepatitis-B vaccines on the day of birth. The USA EPA set a standard of exposure on the safe level of ingested methyl mercury of 0.1 mg/kg body weight. Using this safety level, the newborn would have had to weigh 125 kg to take this exposure safely.”); Haley B., Mercury toxicity: Genetic susceptibility and synergistic effects, Medical Veritas 2 (2005)
535-542 535 (“This data in Figure 2 show that normal children have birth hair levels of mercury that correlate with the number of amalgam fillings in the birth mother; whereas, in sharp contrast, the autistic children have exceptionally low levels of birth hair mercury, no matter what the number of amalgam fillings are found in the birth mother. This data strongly implies that autistic children represent a subset of the population that does not effectively excrete mercury from their cells.”)]
f. Adverse Effects on Kidney Function
Mercury, we now know, concentrates in the kidneys, and experimental evidence shows that it can inhibit kidney function.[67] Distribution of mercury derived from dental amalgam to the kidney was demonstrated by Hahn et al.[68] In this experiment, the organ that accumulated the greatest amount of mercury following amalgam placement was the kidneys.
Scientists are concluding that dental amalgam is an unsuitable restorative material because of its effects on the kidneys. “From the nephrotoxicity point of view, dental amalgam is an unsuitable filling material, as it may give rise to mercury toxicity. In these exposure conditions, renal damage is possible and may be assessed by urinary excretions of albumin, NAG, and gamma-GT.”[69] Additional studies found harm to sheep’s ability to clear inulin a measure of kidney function in just sixty days after implanting mercury/silver fillings.[70]
Critics of the sheep studies claimed that sheep chew too much. Similar studies were conducted on primates (monkeys) fed twice daily and the same distribution pattern for mercury was observed.[71] Animal studies demonstrate exposure to mercury vapor and autoimmunity.[72] One such study showed that dental silver amalgam and silver alloy implanted in the physiological milieu of the peritoneal cavity released enough metals to adversely affect the immune system.[73]
g. Hearing Loss
The effects of amalgam dental fillings on auditory thresholds have been investigated. No significant correlation (p>0.05) was found between composite (non-amalgam) filling or drilling data and auditory thresholds. However, there was a significant positive linear correlation between amalgam fillings and auditory thresholds at 8, 11.2, 12.5, 14, and 16kHz. The strongest association (r=0.587, n=39, p<.001, r(2)=0.345) was at 14kHz, where each additional amalgam filling was associated with a 2.4 dB decline in hearing threshold (95% confidence interval [CI],1.3-3.5 dB).[74]
h. Allergy to Mercury
In the Federal Registry, Volume 52(155):30089, August 12, 1987, the FDA changed the classification of dental mercury, a component part of mercury fillings, from the proposed Class II to Class I, stating, “…warnings under the misbranding provisions (21 U.S.C. 352) of the general controls of the act would warn dentists about the rare risk of allergic reactions among patients and the risk of toxicity to dental health professionals.” Arriving at its conclusion that the risk of allergic reaction was “rare,” the FDA relied on three (3) case reports, ignoring several other scientific studies clearly within the criteria set out in 21 C.F.R. 860.3, 860.7 for valid scientific evidence.
The FDA’s estimation that the risk of allergic reaction is “rare” is undocumented and unscientific. In fact, the scientific literature reflects that between 3.8% and 38.7% of the population with amalgams are allergic to mercury.[75] These studies present formidable evidence that mercury allergy and/or sensitivity is extremely prevalent.
i. Other Adverse Effects
Research has linked mercury from fillings to periodontal disease, inflammation, and bone loss. In addition, research has linked mercury to idiopathic dilated cardiomyopathy (IDCM.) Victims of this disorder may suffer cardiac arrest at an early age. Their hearts have 22,000 times more mercury than comparable hearts that suffered secondary cardiac dysfunction.[76]
Snapp et al in 1981 carefully removed mercury/silver implants and his experimental subjects experienced a dramatic 90% decline in blood mercury.[77] The only logical conclusion is that their mercury/silver implants contributed substantially to their blood mercury. Snapp et al found a dramatic decline in blood mercury while in another similar study, Molin, et al found a dramatic increase followed by a slow drop in blood mercury over the next 12 months to 50% of baseline.[78] The petitioners criticized the careless approach to mercury removal in the Molin et al study, so she repeated the study, with improved and appropriate techniques, confirming Snapp’s earlier finding.[79]
Other adverse health effects associated with mercury exposure are well-documented. Professor Matts Berlin, the World Health Organization’s leading expert on the risks of mercury, recently concluded that: “Regarding the risk for retardation of brain development it is not according to science and standard of care to place amalgam fillings in children and fertile women.”
Furthermore, there is no question that implanting mercury in teeth results in bone loss, and produces inflammation and periodontal breakdown.[80] Thus, as early as 1976, it was apparent that the presence of dental mercury-amalgam resulted in chronic inflammation and bleeding in the gingival tissue adjacent to it; in other words, in situ amalgam produced chronic gingivitis.[81]
In 1984, the year of the NIDR/ADA Workshop, Fisher et al., reported that at amalgam sites alveolar bone loss was very pronounced and statistically significant as compared to control non-amalgam sites.[82] In other words, in situ amalgam produces chronic periodontitis. Periodontal disease is the principal reason for two-thirds of adult tooth loss in the U.S. and mercury from tooth restorations contributes substantially to this common disease.
In 1995, an important review article summarizing some of the scientific documentation concerning dental amalgam was published in the highly prestigious scientific publication, the FASEB Journal. The authors detailed the scientific data and conclusions from scores of peer reviewed articles documenting the deleterious effects of mercury vapor on the immune, renal, reproductive, and central nervous systems. The authors noted that “[r]esearch evidence does not support the notion of amalgam safety.”
In their conclusion, the authors admonished that:
The collective results of numerous research investigations over the past decade clearly demonstrate that the continuous release of Hgº from dental amalgam tooth fillings provides the major contribution to Hg body burden. The experimental evidence indicates that amalgam Hg has the potential to induce cell or organ pathophysiology. At the very least, the traditional dental paradigm, that amalgam is a chemically stable tooth restorative material and that the release of Hg from this material is insignificant, is without foundation. One dental authority states that materials are presently available that are suitable alternatives to Hg fillings. It would seem that now is the time for dentistry to use composite (polymeric and ceramic) alternatives and discard the metal alchemy bestowed on its profession from a less enlightened era. Although human experimental evidence is incomplete at the present time, the recent medical research findings presented herein strongly contradict the unsubstantiated opinions pronounced by various dental associations and related trade organizations, who offer assurances of amalgam safety to dental personnel and their patients without providing hard scientific data, including animal, cellular and molecular evidence, to support their claims.[83]
11. Dental Amalgam is an Implant that Must be in Class III
a. Congress’s Mandate on Classification of Medical and Dental Implants
The Medical and Dental Device Amendments of 1976, 21 U.S.C. §§ 360c, et seq., require FDA to classify dental and medical devices as follows:
(C) In the case of a device which has been referred under paragraph (1) to a panel, and which–
(i) is intended to be implanted in the human body or is purported or represented to be for a use in supporting or sustaining human life, and
(ii)(I) has been introduced or delivered for introduction into interstate commerce for commercial distribution before May 28, 1976, or
(II) is within a type of device which was so introduced or delivered before such date and is substantially equivalent to another device within that type, such panel shall recommend to the Secretary that the device be classified in class III unless the panel determines that classification of the device in such class is not necessary to provide reasonable assurance of its safety and effectiveness. If a panel does not recommend that such a device be classified in class III, it shall in its recommendation to the Secretary for the classification of the device set forth the reasons for not recommending classification of the device in such class.
Amalgam is an implant in the human body and, according to the statutory language should be placed in Class III.
b. FDA Acknowledges that Dental Amalgam is an “Implant”
Until August 4, 2009, dental amalgam was not an FDA approved dental device. There is no FDA notification of approval, no 510K, and no classification of dental amalgam in the Federal Register.
In 1976, Congress directed FDA to evaluate all medical (including dental) devices intended for human use and to classify them according to their safety and effectiveness. [41 FR 34099, August 12, 1976] To this day, “dental amalgam” is not listed as an accepted and classified dental device, even though it has been the most widely utilized of all dental devices.
The FDA Dental Device Division classified “Dental Mercury” as a Class I device, implicitly concluding that this material is safe and effective as a dental device. [52 FR 30082-30108, August 12, 1987] However, FDA thereafter ruled that mercury is not ‘Generally Recognized to be Safe’ (GRAS). [63 FR 19799-19802, April 22, 1998]
Dental amalgam, when utilized as a dental filling material and placed in living tissue in a human body, is a medical/dental device that must be classified under existing law. By definition, it must be classified as an implant and automatically placed in Class III, requiring scientific proof of safety [43 FR 32988, July 28, 1978] The FDA defines “implant” as “a device that is placed into a surgically or naturally formed cavity of the human body. A device is regarded as an implant for the purpose of this part only if it is intended to remain implanted continuously for a period of 30 days or more, unless the commissioner determines otherwise in order to protect human health” [43 FR 32994, July 28, 1978].
In 1978, the FDA Dental Device Panel requested that dental amalgam be exempted from the FDA Rule definition for “implant” [42 FR 46035, Sept. 13, 1977]. The FDA Commissioner denied that request and ruled that mercury fillings were an implant. [43 FR 32988, July 28,1978]
c. Mercury Amalgam Must be Classified in Class III
FDA Rules state: “Although no device can be regulated adequately in Class I or Class II unless there are adequate data and information establishing its safety and effectiveness, a device for which there are such data and information may nevertheless require regulation in Class III because of the public health concerns posed by its use” [42 FR 46030, 13 Sep 1977]. Public health concerns have been repeatedly voiced but ultimately ignored by FDA. The scientific community has long known that mercury is a highly toxic heavy metal, and many prominent scientists have recommended the discontinuation of mercury fillings as a dental restorative material.
On February 20, 2002, FDA announced a proposed rule entitled: “Dental Devices: Classification of Encapsulated Amalgam Alloy and Dental Mercury and Reclassification of Dental Mercury; Issuance of Special Controls for Amalgam Alloy.” The FDA’s announced intention was to reclassify dental mercury into Class II and accept a “capsule” containing dental mercury on one side and amalgam alloy on the other as a “safe and effective” dental device. However, 21 U.S.C. §360c, as well as the agency’s own regulation, 21 C.F.R. § 860.93, requires dental amalgam to be classified into Class III. To be classified in any other class, the Dental Device Panel must file a full statement of the reasons for such classification, including “supporting documentation and data satisfying the requirements of sec. 860.7.” 21 C.F.R. §860.93(b). This regulation provides as follows:
(a) The classification panel will recommend classification into class III of any implant or life-supporting or life-sustaining device unless the panel determines that such classification is not necessary to provide reasonable assurance of the safety and effectiveness of the device. If the panel recommends classification or reclassification of such a device into a class other than class III, it shall set forth in its recommendation the reasons for so doing together with references to supporting documentation and data satisfying the requirements of § 860.7, and an identification of the risks to health, if any, presented by the device.
(b) The Commissioner will classify an implant or life-supporting or life-sustaining device into class III unless the Commissioner determines that such classification is not necessary to provide reasonable assurance of the safety and effectiveness of the device. If the Commissioner proposes to classify or reclassify such a device into a class other than class III, the regulation or order effecting such classification or reclassification will be accompanied by a full statement of the reasons for so doing. A statement of the reasons for not classifying or retaining the device in class III may be in the form of concurrence with the reasons for the recommendation of the classification panel, together with supporting documentation and data satisfying the requirements of § 860.7 and an identification of the risks to health, if any, presented by the device.
In September 2006, a meeting of the Dental Products Panel and the Peripheral and Central Nervous System Drugs Advisory Committee convened to consider, inter alia, whether the conclusions in the FDA’s position statement on amalgam (the “White Paper”) should be deemed “reasonable.” The Joint Panels rejected the FDA contention that the use of dental amalgam may be considered safe. Clearly, no administrative record exists on which the FDA Commissioner or the Dental Device Panel could rationally conclude that there are demonstrable and reasonable assurances that mercury fillings are safe. Amalgam capsules must therefore be classified in Class III.
All, or virtually all, of the references cited herein were submitted with the Citizen’s Petition filed by the IAOMT and DAMS INC. dated July 28, 2025.
F. Certification:
The undersigned certifies, that, to the best knowledge and belief of the undersigned, this petition includes all information and views on which the petition relies, and that it includes representative data and information known to the petitioner which are unfavorable to the petition.
______________________________________
James M. Love
TITUS HILLIS REYNOLDS LOVE, P.C.
- G. M. Richardson et al., “Mercury Exposure and Risks from Dental Amalgam in the US Population, Post-2000,” Sci Total Environ 409 (September 2011): 4257–68, https://doi.org/10.1016/j.scitotenv.2011.06.035. ↑
- Guy Tobias et al., “Survival Rates of Amalgam and Composite Resin Restorations from Big Data Real-Life Databases in the Era of Restricted Dental Mercury Use,” Bioengineering (Basel, Switzerland) 11, no. 6 (2024): 579, https://doi.org/10.3390/bioengineering11060579. ↑
- F. Steenhuisen and S. J. Wilson, “Development and Application of an Updated Geospatial Distribution Model for Gridding 2015 Global Mercury Emissions,” Atmospheric Environment 211 (August 2019): 138–50, https://doi.org/10.1016/j.atmosenv.2019.05.003. ↑
- “Effluent Limitations Guidelines and Standards for the Dental Category,” Federal Register, June 14, 2017, https://www.federalregister.gov/documents/2017/06/14/2017-12338/effluent-limitations-guidelines-and-standards-for-the-dental-category. ↑
- Association of Metropolitan Sewerage Agencies (AMSA), “Mercury Source Control and Pollution Prevention Program Evaluation Final Report (DCN DA00006),” 2002, http://archive.nacwa.org/getfileb882.pdf?fn=finalreport.pdf. ↑
- US EPA, “Technical and Economic Development Document for the Final Effluent Limitations Guidelines and Standards for the Dental Category,” 2016, https://www.epa.gov/sites/production/files/2017-06/documents/dental-office_tedd_dec-2016.pdf. ↑
- L. D. Hylander et al., “High Mercury Emissions from Dental Clinics despite Amalgam Separators,” Sci Total Environ 362 (June 2006): 74–84, https://doi.org/10.1016/j.scitotenv.2005.06.008. ↑
- Federal Register, “Effluent Limitations Guidelines and Standards for the Dental Category.” ↑
- Richardson et al., “Mercury Exposure and Risks from Dental Amalgam in the US Population, Post-2000.” ↑
- Lars Barregard et al., “Renal Effects of Dental Amalgam in Children: The New England Children’s Amalgam Trial,” Environmental Health Perspectives 116, no. 3 (2008): 394–99, https://doi.org/10.1289/ehp.10504. ↑
- U.S. Environmental Protection Agency, Mercury, Elemental; CASRN 7439-97-6 (n.d.), https://iris.epa.gov/ChemicalLanding/&substance_nmbr=370. ↑
- FDA, “List of Patient Preference-Sensitive Priorities; Establishment of a Public Docket; Request for Comments,” May 2019, https://www.regulations.gov/document?D=FDA-2019-N-1619-0001. ↑
- FDA-2019-N-3767, “Regulations.Gov – Notice Document,” 2019, https://www.regulations.gov/document?D=FDA-2019-N-3767-0001. ↑
- Lars Björkman et al., “Perinatal Death and Exposure to Dental Amalgam Fillings during Pregnancy in the Population-Based MoBa Cohort,” PloS One 13, no. 12 (2018): e0208803, https://doi.org/10.1371/journal.pone.0208803. ↑
- Marcelo W. B. Araujo et al., “Amalgam: Impact on Oral Health and the Environment Must Be Supported by Science,” Journal of the American Dental Association (1939) 150, no. 10 (2019): 813–15, https://doi.org/10.1016/j.adaj.2019.07.035. ↑
- The Wealthy Dentist, Does Your Dental Practice Place Amalgam Fillings?, 2008, https://thewealthydentist.com/surveyresults/16_mercuryamalgam_results/. ↑
- E. Bakhurji et al., “Dentists’ Perspective about Dental Amalgam: Current Use and Future Direction,” J Public Health Dent 77 (June 2017): 207–15, https://doi.org/10.1111/jphd.12198. ↑
- “Learned-Intermediary Doctrine Law and Legal Definition | USLegal, Inc.,” accessed July 13, 2025, https://definitions.uslegal.com/l/learned-intermediary-doctrine/. ↑
- G Mark Richardson, ASSESSMENT OF MERCURY EXPOSURE AND RISKS FROM DENTAL AMALGAM, 1995. ↑
- D. A. Geier and M. R. Geier, “Dental Amalgam Fillings and Mercury Vapor Safety Limits in American Adults,” Human & Experimental Toxicology 41 (2022): 9603271221106341, https://doi.org/10.1177/09603271221106341. ↑
- Dan R. Laks, “Assessment of Chronic Mercury Exposure within the U.S. Population, National Health and Nutrition Examination Survey, 1999–2006,” Biometals: An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine 22, no. 6 (2009): 1103–14, https://doi.org/10.1007/s10534-009-9261-0. ↑
- G. Mark Richardson et al., “Mercury Vapour (Hg(0)): Continuing Toxicological Uncertainties, and Establishing a Canadian Reference Exposure Level,” Regulatory Toxicology and Pharmacology: RTP 53, no. 1 (2009): 32–38, https://doi.org/10.1016/j.yrtph.2008.10.004. ↑
- Rosemary Castorina and Tracey J Woodruff, “Assessment of Potential Risk Levels Associated with U.S. Environmental Protection Agency Reference Values.,” Environmental Health Perspectives 111, no. 10 (2003): 1318–25, https://doi.org/10.1289/ehp.6185. ↑
- Richardson et al., “Mercury Vapour (Hg(0)).” ↑
- Jack Schubert et al., “Combined Effects in Toxicology‐a Rapid Systematic Testing Procedure: Cadmium, Mercury, and Lead,” Journal of Toxicology and Environmental Health 4, nos. 5–6 (1978): 763–76, https://doi.org/10.1080/15287397809529698. ↑
- W. D. Ehmann et al., “Brain Trace Elements in Alzheimer’s Disease,” Neurotoxicology 7, no. 1 (1986): 195–206. ↑
- C. M. Thompson et al., “Regional Brain Trace-Element Studies in Alzheimer’s Disease,” Neurotoxicology 9, no. 1 (1988): 1–7. ↑
- D. Wenstrup et al., “Trace Element Imbalances in Isolated Subcellular Fractions of Alzheimer’s Disease Brains,” Brain Research 533, no. 1 (1990): 125–31, https://doi.org/10.1016/0006-8993(90)91804-p. ↑
- S. R. Saxe et al., “Dental Amalgam and Cognitive Function in Older Women: Findings from the Nun Study,” Journal of the American Dental Association (1939) 126, no. 11 (1995): 1495–501, https://doi.org/10.14219/jada.archive.1995.0078. ↑
- Boyd E Haley, The Relationship of the Toxic Effects of Mercury to Exacerbation of the Medical Condition Classified as Alzheimer’s Disease, 2007. ↑
- C. C. Leong et al., “Retrograde Degeneration of Neurite Membrane Structural Integrity of Nerve Growth Cones Following in Vitro Exposure to Mercury,” Neuroreport 12, no. 4 (2001): 733–37, https://doi.org/10.1097/00001756-200103260-00024. ↑
- J. C. Pendergrass et al., “Mercury Vapor Inhalation Inhibits Binding of GTP to Tubulin in Rat Brain: Similarity to a Molecular Lesion in Alzheimer Diseased Brain,” Neurotoxicology 18, no. 2 (1997): 315–24. ↑
- Haley, The Relationship of the Toxic Effects of Mercury to Exacerbation of the Medical Condition Classified as Alzheimer’s Disease. ↑
- J. C. Breitner et al., “Alzheimer’s Disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of Cases, Longitudinal Results, and Observations on Twin Concordance,” Archives of Neurology 52, no. 8 (1995): 763–71, https://doi.org/10.1001/archneur.1995.00540320035011. ↑
- J. T. Ely, “Mercury Induced Alzheimer’s Disease: Accelerating Incidence?,” Bulletin of Environmental Contamination and Toxicology 67, no. 6 (2001): 800–806, https://doi.org/10.1007/s001280193. ↑
- Joachim Mutter et al., “Alzheimer Disease: Mercury as Pathogenetic Factor and Apolipoprotein E as a Moderator,” Neuro Endocrinology Letters 25, no. 5 (2004): 331–39. ↑
- Allen D. Roses and Ann M. Saunders, “Apolipoprotein E Genotyping as a Diagnostic Adjunct for Alzheimer’s Disease,” International Psychogeriatrics 9 (December 1997): 277–88, https://doi.org/10.1017/S1041610297005012. ↑
- D. A. Brouwer et al., “Clinical Chemistry of Common Apolipoprotein E Isoforms,” Journal of Chromatography. B, Biomedical Applications 678, no. 1 (1996): 23–41, https://doi.org/10.1016/0378-4347(95)00256-1. ↑
- Michael E. Godfrey et al., “Apolipoprotein E Genotyping as a Potential Biomarker for Mercury Neurotoxicity,” Journal of Alzheimer’s Disease: JAD 5, no. 3 (2003): 189–95, https://doi.org/10.3233/jad-2003-5303. ↑
- J. C. Pendergrass and Haley. B.E., Inhibition of Brain Tubulin-Guanosine 5’-Triphosphate Interactions by Mercury: Similarity to Observations in Alzheimer’s Diseased Brain, vol. 34, Metal Ions in Biological Systems (Marcel Dekker, Inc., 1996). ↑
- Godfrey et al., “Apolipoprotein E Genotyping as a Potential Biomarker for Mercury Neurotoxicity.” ↑
- Damian P. Wojcik et al., “Mercury Toxicity Presenting as Chronic Fatigue, Memory Impairment and Depression: Diagnosis, Treatment, Susceptibility, and Outcomes in a New Zealand General Practice Setting (1994-2006),” Neuro Endocrinology Letters 27, no. 4 (2006): 415–23. ↑
- Sabiha Khatoon et al., “Aberrant Guanosine Triphosphate–Beta-Tubulin Interaction in Alzheimer’s Disease,” Annals of Neurology 26, no. 2 (1989): 210–15, https://doi.org/10.1002/ana.410260205. ↑
- E. F. Duhr et al., “HgEDTA Complex Inhibits GTP Interactions with the E-Site of Brain Beta-Tubulin,” Toxicology and Applied Pharmacology 122, no. 2 (1993): 273–80, https://doi.org/10.1006/taap.1993.1196; Ehmann et al., “Brain Trace Elements in Alzheimer’s Disease”; Thompson et al., “Regional Brain Trace-Element Studies in Alzheimer’s Disease”; D. E. Vance et al., “Trace Element Imbalances in Hair and Nails of Alzheimer’s Disease Patients,” Neurotoxicology 9, no. 2 (1988): 197–208; Wenstrup et al., “Trace Element Imbalances in Isolated Subcellular Fractions of Alzheimer’s Disease Brains”; Mutter et al., “Alzheimer Disease”; J. T. A. Ely et al., “Urine Mercury in Micromercurialism: Bimodal Distribution and Diagnostic Implications,” Bulletin of Environmental Contamination and Toxicology 63, no. 5 (1999): 553–59, https://doi.org/10.1007/s001289901016; Boyd. E. Haley, Mercury Toxicity: Genetic Susceptibility and Synergistic Effects, 2, no. 2 (2005): 535–42; J. Mutter and F. D. Daschner, “Commentary Regarding the Article by Gottwald et al.: ‘Amalgam Disease’–Poisoning, Allergy, or Psychic Disorder? Int. J. Hyg. Environ. Health 204, 223-229 (2001),” International Journal of Hygiene and Environmental Health 206, no. 1 (2003): 69–70; author reply 71-73, https://doi.org/10.1078/1438-4639-00185; G. Olivieri et al., “Mercury Induces Cell Cytotoxicity and Oxidative Stress and Increases Beta-Amyloid Secretion and Tau Phosphorylation in SHSY5Y Neuroblastoma Cells,” Journal of Neurochemistry 74, no. 1 (2000): 231–36, https://doi.org/10.1046/j.1471-4159.2000.0740231.x; G. Olivieri et al., “The Effects of Beta-Estradiol on SHSY5Y Neuroblastoma Cells during Heavy Metal Induced Oxidative Stress, Neurotoxicity and Beta-Amyloid Secretion,” Neuroscience 113, no. 4 (2002): 849–55, https://doi.org/10.1016/s0306-4522(02)00211-7; Joachim Mutter et al., “Comments on the Article ‘the Toxicology of Mercury and Its Chemical Compounds’ by Clarkson and Magos (2006),” Critical Reviews in Toxicology 37, no. 6 (2007): 537–49; discussion 551-552, https://doi.org/10.1080/10408440701385770; Wojcik et al., “Mercury Toxicity Presenting as Chronic Fatigue, Memory Impairment and Depression”; Pendergrass et al., “Mercury Vapor Inhalation Inhibits Binding of GTP to Tubulin in Rat Brain”; S. David et al., “Abnormal Properties of Creatine Kinase in Alzheimer’s Disease Brain: Correlation of Reduced Enzyme Activity and Active Site Photolabeling with Aberrant Cytosol-Membrane Partitioning,” Brain Research. Molecular Brain Research 54, no. 2 (1998): 276–87, https://doi.org/10.1016/s0169-328x(97)00343-4; C. Hock et al., “Increased Blood Mercury Levels in Patients with Alzheimer’s Disease,” Journal of Neural Transmission (Vienna, Austria: 1996) 105, no. 1 (1998): 59–68, https://doi.org/10.1007/s007020050038; Ely, “Mercury Induced Alzheimer’s Disease.” ↑
- C. H. Ngim and G. Devathasan, “Epidemiologic Study on the Association between Body Burden Mercury Level and Idiopathic Parkinson’s Disease,” Neuroepidemiology 8 (1989): 128–41. ↑
- E. Baasch, “[Theoretical considerations on the etiology of multiple sclerosis. Is multiple sclerosis a mercury allergy?],” Schweizer Archiv Fur Neurologie, Neurochirurgie Und Psychiatrie = Archives Suisses De Neurologie, Neurochirurgie Et De Psychiatrie 98, no. 1 (1966): 1–19. ↑
- W Craelius, “Comparative Epidemiology of Multiple Sclerosis and Dental Caries.,” Journal of Epidemiology and Community Health 32, no. 3 (1978): 155–65, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1060938/. ↑
- T. H. Ingalls, “Epidemiology, Etiology, and Prevention of Multiple Sclerosis. Hypothesis and Fact,” The American Journal of Forensic Medicine and Pathology 4, no. 1 (1983): 55–61, https://doi.org/10.1097/00000433-198303000-00006. ↑
- Ingalls, T.H., “Triggers for Multiple Sclerosis,” Lancet 160 (1986). ↑
- T. H. Ingalls, “Endemic Clustering of Multiple Sclerosis in Time and Place, 1934-1984. Confirmation of a Hypothesis,” The American Journal of Forensic Medicine and Pathology 7, no. 1 (1986): 3–8, https://doi.org/10.1097/00000433-198603000-00002. ↑
- Ahlrot-Westerlund, B., “Multiple Sclerosis and Mercury in Cerebrospinal Fluid,” 1987, 17–21. ↑
- Leszek J. Hahn et al., “Whole-Body Imaging of the Distribution of Mercury Released from Dental Fillings into Monkey Tissues,” The FASEB Journal 4, no. 14 (1990): 3256–60, https://doi.org/10.1096/fasebj.4.14.2227216. ↑
- Robert L. Siblerud and Eldon Kienholz, “Evidence That Mercury from Silver Dental Fillings May Be an Etilological Factor in Multiple Sclerosis,” Science of The Total Environment 142, no. 3 (1994): 191–205, https://doi.org/10.1016/0048-9697(94)90327-1. ↑
- Jenny Stejskal and Vera DM Stejskal, “The Role of Metals in Autoimmunity and the Link to Neuroendocrinology,” Neuroendocrinology Letters, 1999. ↑
- Vera Stejskal et al., “Diagnosis and Treatment of Metal-Induced Side-Effects,” Neuro Endocrinology Letters 27 Suppl 1 (December 2006): 7–16; Vera Stejskal et al., “Metal-Induced Inflammation Triggers Fibromyalgia in Metal-Allergic Patients,” Neuro Endocrinology Letters 34, no. 6 (2013): 559–65. ↑
- Ely et al., “Urine Mercury in Micromercurialism.” ↑
- K. G. Homme et al., “New Science Challenges Old Notion That Mercury Dental Amalgam Is Safe,” Biometals 27 (February 2014): 19–24, https://doi.org/10.1007/s10534-013-9700-9. ↑
- I. A. Brown, “Chronic Mercurialism; a Cause of the Clinical Syndrome of Amyotrophic Lateral Sclerosis,” A.M.A. Archives of Neurology and Psychiatry 72, no. 6 (1954): 674–81. ↑
- A. D. Kantarjian, “A Syndrome Clinically Resembling Amyotrophic Lateral Sclerosis Following Chronic Mercurialism,” Neurology 11 (July 1961): 639–44, https://doi.org/10.1212/wnl.11.7.639. ↑
- T. E. Barber, “Inorganic Mercury Intoxication Reminiscent of Amyotrophic Lateral Sclerosis,” Journal of Occupational Medicine.: Official Publication of the Industrial Medical Association 20, no. 10 (1978): 667–69. ↑
- C. R. Adams et al., “Mercury Intoxication Simulating Amyotrophic Lateral Sclerosis,” JAMA 250, no. 5 (1983): 642–43. ↑
- Y. Mano et al., “[Amyotrophic lateral sclerosis and mercury–preliminary report],” Rinsho Shinkeigaku = Clinical Neurology 30, no. 11 (1990): 1275–77. ↑
- O. Redhe and J. Pleva, “Recovery from Amyotrophic Lateral Sclerosis and from Allergy after Removal of Dental Amalgam Fillings,” The International Journal of Risk & Safety in Medicine 4, no. 3 (1994): 229–36, https://doi.org/10.3233/JRS-1994-4307. ↑
- S. Schwarz et al., “Amyotrophic Lateral Sclerosis after Accidental Injection of Mercury,” Journal of Neurology, Neurosurgery, and Psychiatry 60, no. 6 (1996): 698, https://doi.org/10.1136/jnnp.60.6.698. ↑
- S. S. Khare et al., “Trace Element Imbalances in Amyotrophic Lateral Sclerosis,” Neurotoxicology 11, no. 3 (1990): 521–32. ↑
- David Geier et al., “A Prospective Study of Prenatal Mercury Exposure from Maternal Dental Amalgams and Autism Severity,” Acta Neurobiologiae Experimentalis 69, no. 2 (2009): 2, https://doi.org/10.55782/ane-2009-1744. ↑
- N. D. Boyd et al., “Mercury from Dental ‘Silver’ Tooth Fillings Impairs Sheep Kidney Function,” The American Journal of Physiology 261, no. 4 Pt 2 (1991): R1010-1014, https://doi.org/10.1152/ajpregu.1991.261.4.R1010. ↑
- L. J. Hahn et al., “Dental ‘Silver’ Tooth Fillings: A Source of Mercury Exposure Revealed by Whole-Body Image Scan and Tissue Analysis,” FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 3, no. 14 (1989): 2641–46, https://doi.org/10.1096/fasebj.3.14.2636872. ↑
- Wael L Mortada et al., “Mercury in Dental Restoration: Is There a Risk of Nephrotoxicity,” Journal of Nephrology 15, no. 2 (2002): 171–76. ↑
- Boyd et al., “Mercury from Dental ‘Silver’ Tooth Fillings Impairs Sheep Kidney Function.” ↑
- Hahn et al., “Whole-Body Imaging of the Distribution of Mercury Released from Dental Fillings into Monkey Tissues.” ↑
- K. Warfvinge et al., “Systemic Autoimmunity Due to Mercury Vapor Exposure in Genetically Susceptible Mice: Dose-Response Studies,” Toxicology and Applied Pharmacology 132, no. 2 (1995): 299–309, https://doi.org/10.1006/taap.1995.1111. ↑
- Per Hultman et al., “Adverse Immunological Effects and Autoimmunity Induced by Dental Amalgam and Alloy in Mice,” The FASEB Journal 8, no. 14 (1994): 1183–90, https://doi.org/10.1096/fasebj.8.14.7958626. ↑
- Janet A. Rothwell and Paul J. Boyd, “Amalgam Dental Fillings and Hearing Loss,” International Journal of Audiology 47, no. 12 (2008): 770–76, https://doi.org/10.1080/14992020802311224. ↑
- Tomio Mori et al., “Positive Patch Test for Mercury Possibly from Exposure to Amalgam,” Environmental Health and Preventive Medicine 12, no. 4 (2007): 172–77, https://doi.org/10.1007/BF02897987; E. G. Miller et al., “Prevalence of Mercury Hypersensitivity in Dental Students,” The Journal of Prosthetic Dentistry 58, no. 2 (1987): 235–37, https://doi.org/10.1016/0022-3913(87)90183-1; R. R. White and R. L. Brandt, “Development of Mercury Hypersensitivity among Dental Students,” Journal of the American Dental Association (1939) 92, no. 6 (1976): 1204–7, https://doi.org/10.14219/jada.archive.1976.0168; Susann Forkel et al., “Contact Allergies to Dental Materials in Patients,” The British Journal of Dermatology 190, no. 6 (2024): 895–903, https://doi.org/10.1093/bjd/ljad525; Inger M. C. Lundström, “Allergy and Corrosion of Dental Materials in Patients with Oral Lichen Planus,” International Journal of Oral Surgery 13, no. 1 (1984): 16–24, https://doi.org/10.1016/S0300-9785(84)80051-4; Kaj Finne et al., “Oral Lichen Planus and Contact Allergy to Mercury,” International Journal of Oral Surgery 11, no. 4 (1982): 236–39, https://doi.org/10.1016/S0300-9785(82)80073-2. ↑
- A. Frustaci et al., “Marked Elevation of Myocardial Trace Elements in Idiopathic Dilated Cardiomyopathy Compared with Secondary Cardiac Dysfunction,” Journal of the American College of Cardiology 33, no. 6 (1999): 1578–83, https://doi.org/10.1016/s0735-1097(99)00062-5. ↑
- K. R. Snapp et al., “The Contribution of Dental Amalgam to Mercury in Blood,” Journal of Dental Research 68, no. 5 (1989): 780–85, https://doi.org/10.1177/00220345890680050501. ↑
- Snapp et al., “The Contribution of Dental Amalgam to Mercury in Blood”; M. Molin, “Mercury Release from Dental Amalgam in Man. Influences on Selenium, Glutathione Peroxidase and Some Other Blood and Urine Components,” Swed Dent J Suppl 71 (1990): 1–122. ↑
- M. Molin, “Kinetics of Mercury in Blood and Urine after Amalgam Removal.,” J Dent Res 74 (1995): 420. ↑
- A. R. Pack et al., “The Prevalence of Overhanging Margins in Posterior Amalgam Restorations and Periodontal Consequences,” Journal of Clinical Periodontology 17, no. 3 (1990): 145–52, https://doi.org/10.1111/j.1600-051x.1990.tb01078.x; Helen McParland and Saman Warnakulasuriya, “Oral Lichenoid Contact Lesions to Mercury and Dental Amalgam—A Review,” Journal of Biomedicine and Biotechnology 2012 (2012): 589569, https://doi.org/10.1155/2012/589569; H. A. Zander, “Effect of Silicate Cement and Amalgam on the Gingiva,” The Journal of the American Dental Association 55, no. 1 (1957): 11–15, https://doi.org/10.14219/jada.archive.1957.0142; George R. App, “Effect of Silicate, Amalgam, and Cast Gold on the Gingiva,” The Journal of Prosthetic Dentistry 11, no. 3 (1961): 522–32, https://doi.org/10.1016/0022-3913(61)90235-9; L. S. Sotres et al., “A Histologic Study of Gingival Tissue Response to Amalgam, Silicate and Resin Restorations,” Journal of Periodontology 40, no. 9 (1969): 543–46, https://doi.org/10.1902/jop.1969.40.9.543; S. C. Trivedi and S. T. Talim, “The Response of Human Gingiva to Restorative Materials,” The Journal of Prosthetic Dentistry 29, no. 1 (1973): 73–80, https://doi.org/10.1016/0022-3913(73)90142-x. ↑
- PaulR. Goldschmidt et al., “Effects of Amalgam Corrosion Products on Human Cells,” Journal of Periodontal Research 11, no. 2 (1976): 108–15, https://doi.org/10.1111/j.1600-0765.1976.tb00058.x. ↑
- D. Fisher et al., “A 4 Year Follow-up Study of Alveolar Bone Height Influenced by Two Dissimilar Class II Amalgam Restorations,” Journal of Oral Rehabilitation 11, no. 4 (1984): 399–405, https://doi.org/10.1111/j.1365-2842.1984.tb00592.x. ↑
- F. L. Lorscheider et al., “Mercury Exposure from ‘Silver’ Tooth Fillings: Emerging Evidence Questions a Traditional Dental Paradigm,” FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 9, no. 7 (1995): 504–8. ↑


